The forgotten people: Hepatitis B virus (HBV) infection as a priority for the inclusion health agenda
Abstract
Hepatitis B virus (HBV) infection represents a significant global health threat, accounting for 300 million chronic infections and up to 1 million deaths each year. HBV disproportionately affects people who are under-served by health systems due to social exclusion, and can further amplify inequities through its impact on physical and mental health, relationship with stigma and discrimination, and economic costs. The ‘inclusion health’ agenda focuses on excluded and vulnerable populations, who often experience barriers to accessing healthcare, and are under-represented by research, resources, interventions, advocacy, and policy. In this article, we assimilate evidence to establish HBV on the inclusion health agenda, and consider how this view can inform provision of better approaches to diagnosis, treatment, and prevention. We suggest approaches to redress the unmet need for HBV interventions among excluded populations as an imperative to progress the global goal for the elimination of viral hepatitis as a public health threat.
Introduction
Hepatitis B virus (HBV) infection is estimated to account for 300 million chronic infections and 1 million deaths each year (World Health Organization, 2021). The global burden of HBV infection is unevenly distributed, with particularly high prevalence in some populations in Africa and South East Asia (Cooke et al., 2019). Likewise, HBV is not experienced equally across society, with a disproportionate prevalence and impact on marginalised and deprived populations, whose needs are poorly met by existing healthcare research, services, interventions and policies (O’Hara et al., 2017; Figure 1A). The ‘inclusion health’ agenda focuses on these populations, setting out to identify and address health and social inequities, for example barriers to accessing mainstream healthcare services (Royal College of Physicians, 2022; Aldridge et al., 2018). These groups include, but are not limited to, migrants in vulnerable situations, people experiencing homelessness (PEH), individuals with substance use disorders, commercial sex workers (CSW), incarcerated individuals, and Roma and Traveller communities (Royal College of Physicians, 2022).
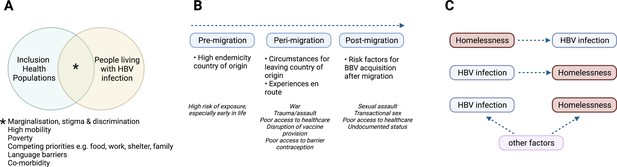
Characteristics of HBV in inclusion health populations.
(A) Illustration of the overlapping characteristics that may be present among different inclusion populations and people living with HBV infection. (B) Relationship between migrancy and asylum-seeking status as a risk factor for HBV infection. (C) Representation of complex relationship between HBV infection and PEH, where other factors include for example injecting drug use, transactional sex, mental illness (Freeland et al., 2021; Ly et al., 2021a). HBV – hepatitis B virus; PEH - People experiencing homelessness. Figure created in BioRender.com with a licence to publish.
Viral hepatitis is recognised as a global health priority within the United Nations Sustainable Development Goals 2030 (SDG30). These underpin ambitious targets including 90% HBV vaccine coverage, reducing new chronic infections and new infections by 90% and attributable mortality by 65% (Cooke et al., 2019). Vaccination against HBV, including universal birth dose administration, is a key elimination strategy and is estimated to have prevented 310 million cases of HBV infection between 1990 and 2020 (Cooke et al., 2019, de Villiers et al., 2021). However, birth dose vaccine implementation has been slow in reaching the most vulnerable populations, while a three-dose vaccine schedule remains a challenge (de Villiers et al., 2021). Lack of education and awareness, inadequate living conditions, lack of access to healthcare services, and poverty and stigma among marginalised populations create a permissive environment for HBV transmission. Chronic HBV infection usually remains asymptomatic until late complications (liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC)). Silent infection, in combination with barriers to testing in the most affected populations, explains why only an estimated 10% of those living with HBV infection are diagnosed (O’Hara et al., 2017).
There is no cure for HBV, but antivirals (nucleos(t)ide analogue agents) can suppress viral replication. Based on international treatment guidelines, only a minority of those with chronic infection are eligible for treatment, and a small proportion of these can actually access consistent therapy (McNaughton et al., 2021). Risk stratification requires consistent surveillance (including clinical review, imaging and laboratory tests), but this can feel burdensome and irrelevant for some people living with asymptomatic infection and/or those who do not meet treatment criteria (Mofokeng, 2021, Adjei et al., 2019). Moreover, HBV receives only a fraction of global research funding allocated to major infectious diseases. For example, in 2022, projected US funding for HIV research is 20-fold greater than for HBV, despite the lower number of people living with HIV infection (National institutes of health, 2022). Therefore, HBV remains a major public health threat, with 1.5 million new infections each year and more annual deaths than malaria or HIV (O’Hara et al., 2017, Graber-Stiehl, 2018).
An advocate with lived experience of HBV infection described the HBV community as ‘the forgotten people’, a description that is also pertinent to inclusion health populations (Matthews et al., 2022a, Aldridge et al., 2018). These exclusions may lead to an increased risk of HBV infection, are amplified by HBV infection, and lead to greater morbidity and mortality as a result of inadequate access to care. In resource-limited settings, where the burden of HBV infection is greatest, the inequities are amplified.
We have collated evidence for the impact of HBV in some of these marginalised populations globally, assimilating data and identifying knowledge gaps in order to inform the HBV agenda in inclusion health. Addressing the complex intersection of social exclusion and HBV infection is essential to support progress towards global elimination goals.
HBV infection in inclusion health populations
In this section, we review data for different populations who are frequently marginalised and under-served by healthcare provision. The characteristics, experiences and needs of these groups are diverse, but also have features in common, and approaches should be integrated as far as possible.
Migrants
By the end of 2020, there were 281 million international migrants, equivalent to 3.6% of the world’s population (International organisation for migration, 2021). A significant proportion (approaching 90 million) are displaced due to conflict, political instability, and natural disasters. In Europe, more than half of 49 million migrants born outside of the European Economic Area came from nations where HBV endemicity is either intermediate (2–8%) or high (>8%), explaining the higher prevalence of HBV infection in migrants (5%), and asylum seekers (10%) compared to the general population (1%) in Europe (European Centre for Disease Prevention and Control, 2016; Kim et al., 2021; Table 1). Descriptive studies from Italy and the UK provide some insight into HBV infection among migrants and unaccompanied asylum-seeking children (Table 2; Prestileo et al., 2022, Colucci et al., 2022; Mazzitelli et al., 2021; Armitage et al., 2022; Williams et al., 2020a, World Health Organization, 2002), reporting risks of infection that apply before, during and after relocation (Figure 1). In some countries, including the UK, asylum seekers may be moved hundreds of miles away at short notice, disrupting any continuity of care (Farrant et al., 2022; World Health Organization, 2018b).
Top 20 origins of international migrants in 2020 (millions), HBV prevalence and progress towards SDG 30 goals for elimination of HBV as a public health threat.
Data - United Nations Department of Economic and Social Affairs, Population Division (2020b). International Migrant Stock 2020. https://www.un.org/development/desa/pd/content/international-migrant-stock; The Polaris Observatory, CDA Foundation; https://cdafound.org/polaris-countries-compare/. HBV – Hepatitis B virus. SDG – Sustainable Development Goal.
| Country of Origin | Number of International Migrants (millions) | HBV Prevalence* | 90% Diagnosed† | 80% Treated† | 65% Reduction in Mortality† | Reduced Prevalence in 5 year olds† |
|---|---|---|---|---|---|---|
| India | 17.9 | 3% | 2051 | 2051 | 2051 | 2032 |
| Mexico | 11.2 | 0% | 2051 | 2051 | 2051 | 2015 |
| Russia | 10.8 | 1% | 2051 | 2051 | 2051 | 2015 |
| China | 10.5 | 6% | 2051 | 2051 | 2051 | 2021 |
| Syria | 8.5 | 6% | 2051 | 2051 | 2051 | 2051 |
| Bangladesh | 7.4 | 5% | 2051 | 2051 | 2051 | 2043 |
| Pakistan | 6.3 | 1% | 2042 | 2051 | 2051 | 2036 |
| Ukraine | 6.1 | 1% | 2051 | 2051 | 2051 | 2030 |
| Philippines | 6.1 | 10% | 2051 | 2051 | 2051 | 2051 |
| Afghanistan | 5.9 | 3% | 2051 | 2051 | 2051 | 2045 |
| Venezuela | 5.4 | 1% | 2051 | 2051 | 2051 | 2031 |
| Poland | 4.8 | 1% | 2051 | 2051 | 2051 | 2015 |
| United Kingdom | 4.7 | 1% | 2051 | 2051 | 2051 | 2020 |
| Indonesia | 4.6 | 7% | 2051 | 2051 | 2051 | 2051 |
| Kazakhstan | 4.2 | 4% | 2051 | 2051 | 2051 | 2027 |
| Palestine | 4.0 | 2% | 2051 | 2051 | 2051 | 2015 |
| Romania | 4.0 | 3% | 2051 | 2051 | 2051 | 2025 |
| Germany | 3.9 | 0% | 2039 | 2051 | 2051 | 2015 |
| Myanmar | 3.7 | 8% | 2051 | 2051 | 2051 | 2051 |
| Egypt | 3.6 | 1% | 2051 | 2051 | 2051 | 2018 |
-
*
Green -Low HBV prevalence (<2%); Amber - intermediate HBV prevalence (2–8%); Red - high HBV (prevalence >8%).
-
†
Green - HBV SDG reached before 2030; Amber - SDG reached 2031–50; Red - SDG reached >2050.
Key review and study observations pertinent to HBV infection among inclusion health populations.
| Inclusion health population | Citation | Study type | Country | Key observations |
|---|---|---|---|---|
| PEH, PWID, Incarcerated individuals, sex workers | Aldridge et al., 2018 | Systematic Review and Meta-analysis | High-income countries |
|
| PWID, PEH, Previous incarceration | Taylor et al., 2019 | Cross-sectional | UK |
|
| Migrants | Prestileo et al., 2022 | Cross-sectional | Italy |
|
| Colucci et al., 2022 | Cross-sectional | Italy | Increased risk of BBV acquisition persisted after arrival in Italy, possibly due to living conditions, sex work, lack of access to healthcare and social support | |
| Mazzitelli et al., 2021 | Cross-sectional | Italy |
| |
| Armitage et al., 2022 | Cross-sectional | UK |
| |
| Williams et al., 2020a | Cross-sectional | UK |
| |
| Eborall et al., 2020 | Qualitative | UK |
| |
| Tasa et al., 2021 | Retrospective cohort | Finland |
| |
| Bierhoff et al., 2021 | Mixed-methods | Thailand |
| |
| PEH | Ly et al., 2021a | Narrative review | Global |
|
| Al Shakarchi et al., 2020 | Systematic review and meta-analysis | Global |
| |
| PWID | Degenhardt et al., 2017 | Multistage systematic review | Global |
|
| People who misuse alcohol | Magri et al., 2020 | Systematic review and meta-analysis | Global |
|
| Incarcerated people | Dolan et al., 2016 | Systematic review and meta-analysis | Global |
|
| Nakitanda et al., 2021 | Descriptive analysis | Europe |
| |
| Kamarulzaman et al., 2016 | Narrative review | Global |
| |
| Dana et al., 2013 | Cross-sectional | Iran |
| |
| Sex workers | Schuelter-Trevisol et al., 2013 | Cross-sectional | Brazil |
|
| Miranda et al., 2021 | Cross-sectional | Brazil |
| |
| Matos et al., 2017 | Cross-sectional | Brazil |
| |
| Leuridan et al., 2005 | Cross-sectional | Belgium |
| |
| Mak et al., 2003 | Cross-sectional | Belgium |
| |
| Dos Ramos Farías et al., 2011 | Cross-sectional | Argentina |
| |
| Todd et al., 2010 | Cross-sectional | Afghanistan |
| |
| Jeal and Salisbury, 2004 | Cross-sectional | UK |
| |
| Bitty-Anderson et al., 2021 | Cross-sectional | Togo |
| |
| Roma and Traveller populations | Macejova et al., 2020 | Cross-sectional | Slovakia |
|
| Gregory et al., 2014 | Cross-sectional | UK |
| |
| Veselíny et al., 2014 | Cross-sectional | Slovakia |
| |
| Indigenous Populations | Davies et al., 2019 | Cross-sectional | Australia |
|
| Qama et al., 2021 | Retrospective cohort | Australia |
| |
| Einsiedel et al., 2013 | Retrospective cohort | Australia |
| |
| Osiowy et al., 2013 | Narrative review | USA, Canada, Greenland |
| |
| Russell et al., 2019 | Systematic review | Latin America |
|
-
SMR Standardised Mortality Ratio; aOR adjusted odds ratio; OR odds ratio; RR relative risk; CI confidence interval; HBV hepatitis B virus; UI Uncertainty Interval; MSM men who have sex with men; PEH people experiencing homelessness, PWID people who inject drugs; UASC unaccompanied asylum-seeking children; HBsAg Hepatitis B surface antigen (active HBV infection); HBc Hepatitis B core antibody
People experiencing homelessness
Approximately 150 million people worldwide were considered homeless in 2020 (Chamie, 2017). People experiencing homelessness (PEH) endure extreme social exclusion and have substantially increased mortality from any cause compared to a socially deprived population (Table 2; Aldridge et al., 2018). Seroprevalence surveys, although of varying size and quality, report higher HBV seroprevalence among PEH compared to the housed population (Ly et al., 2021a). Homelessness may be a risk factor for HBV infection or vice versa, while there are also shared risk factors which include associations with place of birth, older age, sexual partners with a history of hepatitis, and injecting drug or alcohol use (Ly et al., 2021a, Ly et al., 2021b; Figure 1C). Low HBV vaccination uptake among PEH may relate to either never being offered the vaccine, or being unable to access follow-up doses (Ly et al., 2021b, Taylor et al., 2019).
Among PEH, fear of judgement and stigma relating to homelessness can lead to distrust of healthcare workers and services, basic needs (food, clothing and shelter) are prioritised over long-term healthcare needs, and additional barriers are common (lack of insurance, transport costs, and long waiting times in unfamiliar environments) (Crone et al., 2022; Becker and Foli, 2022; Paisi et al., 2022). High prevalence of co-morbid conditions compounds social exclusion, for example severe mental illness or substance use disorders (Shepherd-Banigan, 2021).
People who misuse drugs and alcohol
It is estimated that there are 15.6 million People Who Inject Drugs (PWID) between the ages of 15–64 years worldwide (Degenhardt et al., 2017), while an estimated 283 million people have alcohol use disorders (World Health Organization, 2018a). HBV infection disproportionately affects people who misuse drugs and alcohol (Table 2; Degenhardt et al., 2017; Magri et al., 2020). Alcohol use is associated with increased high-risk sexual activity, drug use, and sharing injecting equipment, and therefore may increase risk of HBV transmission (Arasteh et al., 2008), while alcohol acts synergistically with HBV infection to accelerate liver damage (Gitto et al., 2009; Li et al., 2019).
Perceived lack of need, competing priorities (e.g. avoiding opioid withdrawal), discrimination, stigma and difficulty in navigating health systems are reasons that PWID and people with alcohol use disorder may avoid seeking care (Motavalli et al., 2021; Zewdu et al., 2019; Costa et al., 2020). Inadequate funding for harm reduction and addiction programmes is a barrier to HBV prevention. Criminalisation and imprisonment of PWID exacerbates transmission and creates further barriers to consistent HBV care provision (O’Keefe et al., 2017). Finally, a fifth of PWID have experienced recent homelessness (within 1 year) and over two-thirds have a history of incarceration (Degenhardt et al., 2017; Rashti et al., 2020). This intersection of overlapping risks further amplifies HBV infection and increases the challenges for healthcare.
Incarcerated people
At the end of 2021, the worldwide prison population was at least 10.77 million (Fair and Walmsley, 2021). The majority of published HBV literature representing prisons consists of small cross sectional seroprevalence studies, which do not reflect the dynamic nature of prison populations (Wirtz et al., 2018; Dolan et al., 2016). Nevertheless, HBV is over-represented in incarcerated individuals, with an estimated global HBsAg prevalence of 4.8% (Dolan et al., 2016; Nakitanda et al., 2021).
Prisons create a complex intersection of individual, social and environmental challenges, and have been described as a ‘concentration mechanism’ leading to amplification and dissemination of infectious diseases (Kamarulzaman et al., 2016), particularly when injecting drug use and sex work are criminalised. 25% of PWID report initiating drug use in prisons and incarceration is associated with a doubled risk of re-initiating injecting drug use (Genberg et al., 2015; Kamarulzaman et al., 2016). Sharing contaminated needles for injecting drugs, sharing tooth brushes, unsterile tattooing, body piercing and high-risk sexual activity also represent potential routes of HBV transmission within prisons (Kamarulzaman et al., 2016; Moazen et al., 2018; Kowo et al., 2021; Harawa et al., 2010). This risk continues beyond prison; the immediate period following release is associated with high risk of heightened sexual behaviours, and drug/alcohol use (Kamarulzaman et al., 2016; Dolan et al., 2016; Binswanger et al., 2007). Frequency and duration of incarceration are also positively associated with prevalence of HBV infection (Dana et al., 2013). Given the overlap of this population and many other HBV risk factors, this is a particularly important target group in which HBV interventions need to be carefully focused, both during and after incarceration periods.
People who engage in transactional sex
This group is highly heterogenous, representing commercial sex workers (CSW) but also a wider group of individuals practising intermittent transactional sex (in exchange for goods, drugs, accommodation etc), overlapping with PWID and PEH populations (Table 2). Sex workers are underrepresented in the literature and robust data are lacking (Aldridge et al., 2018). HBV prevalence varies between countries and sex worker populations; cross-sectional data from Brazil suggest regional differences in HBV seroprevalence (Schuelter-Trevisol et al., 2013; Miranda et al., 2021; Matos et al., 2017), and higher risk groups include male sex workers and trans-female sex workers (Leuridan et al., 2005; Dos Ramos Farías et al., 2011; Mak et al., 2003). Older age, marital status, having children, fewer years in education, drug and alcohol use, meeting clients on the street and high numbers of clients are associated with HBV exposure among CSWs (Miranda et al., 2021; Matos et al., 2017; Todd et al., 2010).
A study of street-based female sex workers (FSW) in the UK found that most did not disclose their work to a primary care practitioner, and in pregnancy, 13% sought antenatal services for the first time whilst in labour, reducing opportunities to prevent mother to child transmission of HBV. Barriers to care for FSW include waiting times, fear of judgement and travelling long distances for healthcare (Jeal and Salisbury, 2004; Mc Grath-Lone et al., 2014).
Roma and Traveller communities
These communities encompass diverse distinct cultural and ethnic groups, also including travelling show people and boat dwellers. In Europe, these populations number 10–12 million, but this is likely to be an underestimate due to high mobility and poor data capture (Council of Europe, 2020). These communities experiences extreme health disparities, with a 10-year lower life expectancy than the settled population in the UK (Equality and Human Rights Commission, 2017). HBV is not well studied among these populations, but in the UK a study of Roma people found a HBV surface antigen (HBsAg) prevalence of 9.4% and in Eastern Slovakia, Roma community members were four times more likely to be HBsAg-positive compared to the age-matched general population (Macejova et al., 2020; Gregory et al., 2014). Risk factors associated with HBV infection included being male, older age, tattoos, and previous imprisonment (Veselíny et al., 2014).
Mainstream healthcare services are not inclusive towards the lifestyle and culture of travelling communities, with barriers arising through discrimination, alongside lack of fixed address and low levels of literacy (McFadden et al., 2018). Belief in God’s will, prioritising work and family commitments over personal health, mistrust in healthcare services and everyday stigma likely contribute to underdiagnosis, undertreatment and lack of preventative measures for HBV infection (Prestileo et al., 2022).
Indigenous communities
Indigenous populations, a global term referring to people with historical ties to a land prior to colonisation, are disproportionately affected by HBV (Osiowy et al., 2013; Russell et al., 2019; Howell et al., 2019). For example, Aboriginal Australians have a higher HBV prevalence, higher rate of liver disease, and poorer outcomes, compared to non-Indigenous Australians (Plackett, 2022; Qama et al., 2021; Wigg et al., 2021). A variety of factors may account for this disparity, including reduced healthcare access due to remote location, social inequalities and multi-morbidity (Plackett, 2022; Einsiedel et al., 2013). HBV genotype may also be an important consideration; genotype C4 in Aboriginal Australians is associated with aggressive disease progression, however this impact is yet to be unpicked from the effects of social determinants of health (Davies et al., 2019; Plackett, 2022). In addition, there is some evidence that current HBV vaccine, which is designed against genotype A, may have reduced efficacy against C4 (Qama et al., 2021). Although culturally and geographically distinct, indigenous populations across the globe face an increased burden and worse outcomes from HBV, and are poorly represented by existing data. Enhanced research, allocation of resources, services, educations and intervention is required to reduce inequities.
Sex and gender as risk factors for HBV infection
Within inclusion health populations, evidence suggests that females with HBV fare worse than males (Table 2). For example, HBV infection was twice as likely in female migrants in Italy, and a higher proportion of female migrants and unaccompanied asylum-seeking children report a history of sexual assault/abuse which represents a significant HBV transmission risk (Prestileo et al., 2022, Armitage et al., 2022). Being female was associated with lower HBV vaccination uptake in PEH, PWID and people with a history of incarceration in the UK (Taylor et al., 2019).
It could be argued that HBV presents a ‘double jeopardy’ in females, given the risk of both individual disease and mother-to-child transmission. A Finnish study found that HBV prevalence in pregnancy was significantly higher in undocumented migrants than in the general population. Most pregnant migrants entered antenatal care late, and either received inadequate care or none at all (Tasa et al., 2021). Education and awareness are also lacking among women in some inclusion health populations: pregnant migrant women in Thailand had very low levels of HBV knowledge (Bierhoff et al., 2021), and despite a high prevalence of HBV (8%), more than 90% of FSW in Mumbai had not heard of HBV infection (United Way Mumbai, 2017).
This female disadvantage observed in inclusion health populations with HBV is reflected in the dramatically increased all-cause mortality in females compared to males across the wider inclusion health population, with female standardised mortality ratio (SMR) 11.86 (95% CI 10.42–13.3; I2 91.5%) vs. Male SMR 7.88 (95% CI 6.40–9.37; I2 98.1%) (Aldridge et al., 2018).
These observations within inclusion health populations contrast with observations from the wider population, which suggest males have worse HBV outcomes than females, with higher rates of exposure, chronicity and complications (Brown et al., 2022). Further work is needed to determine whether this sexual dimorphism is truly reversed in HBV within inclusion health populations, and highlights the importance of considering HBV within the sociodemographic context of inclusion health populations as this may affect risk stratification and targeted elimination strategies.
HBV and the Inclusion Health Agenda: A global perspective
The majority of existing research on inclusion health and HBV is biased towards high-income settings such as Europe and North America (Table 2). Gathering relevant data in some high endemicity countries, including countries in the WHO African, South-East Asian and Western Pacific regions is made more difficult due to criminalisation and/or stigmatisation of inclusion health populations for example CSW, PWID and LGBTQ+ communities. Depending on the sampling methods, available data suggest intermediate to high HBV prevalence in CSWs, PWID, and incarcerated people in African populations (Bitty-Anderson et al., 2021; Degenhardt et al., 2017; Dolan et al., 2016; Scheibe et al., 2020). Fear of discrimination by healthcare workers, possible legal consequences and refusal of services are just some of the barriers deterring inclusion health populations from seeking care and remaining in longer term follow up (Duby et al., 2018). Increased difficulties collecting relevant data, heightened exclusion from healthcare services and increased HBV prevalence, mean it is of even greater importance to prioritise HBV in the inclusion health agenda globally, particularly in Africa where HBV research, advocacy, representation and investment have been neglected.
Enhancing HBV within the Inclusion Health Agenda: The next steps
In this section, we consider how the current evidence informs policy and practical strategies to advance prevention, diagnosis, clinical care and treatment for people living with HBV infection in groups who have been under-served to date.
Close the data gap
There are clear SDG30 goals for HBV, but it is impossible to determine the nature and magnitude of the challenge, benchmark progress, or deploy appropriate interventions without accurate epidemiological data. European Centre for Disease Prevention and Control report major data gaps, now exacerbated by the Covid-19 pandemic, with the worst impact in high-burden (often low income) settings, hampering progress towards elimination (Pley et al., 2021). Good quality HBV data for inclusion health populations are lacking, mostly reporting from small, independent, cross sectional seroprevalence studies (summarised in Table 2). Unified, transparent data collection will require increased awareness, advocacy, funding and research, with coordination between organisations (e.g. World Health Organization, United States Centers for Disease Control and Prevention, European Centre for Disease Prevention and Control) to help standardise and strengthen approaches. Crucially, evidence is needed to underpin solution-based methods rather than simply describing problems (Luchenski et al., 2018; Siersbaek et al., 2021).
Active case finding
Active targeted screening efforts are an important step towards HBV elimination (European Centre for Disease Prevention and Control, 2021; Lemoine et al., 2015). Availability of point-of-care hepatitis B surface antigen tests enable community-based diagnosis, although global efforts are needed to prioritise regular supply in resource-constrained settings (Lemoine et al., 2015). For migrants, community-based testing strategies are effective, with highest uptake in programmes with endorsement from the local community (European Centre for Disease Prevention and Control, 2018; Robotin and George, 2014). Outreach screening is equally important in other inclusion health populations, for example, PEH and PWID (Box 1). Such outreach screening services have been shown to be feasible and acceptable (O’Carroll et al., 2017, Carr et al., 2014), and can also be incorporated into other services (e.g. drug harm reduction programmes).
Case studies of inclusion health interventions.
1. RESPOND (Farrant et al., 2022): RESPOND is a team from University College London Hospitals in the London borough of Camden, providing rapid access community based integrated screening and care planning for asylum seekers. It was formed to accommodate the needs of the rising number of people seeking asylum in temporary accommodation at the beginning of the Covid-19 pandemic. The aim is to provide a multi-disciplinary, comprehensive assessment and formulate an integrated migrant health plan, an electronic document that outlines the key issues for each family member. This document remains with the family, and is copied to the primary healthcare provider, thus removing the need for repeated assessments after each short notice relocation.
2. Find & Treat (University College London Hospitals NHS Foundation Trust, 2013): University College London Hospitals Find & Treat are a specialist outreach team working alongside over 200 NHS and third sector front-line services to tackle TB and blood borne viruses among PEH, PWID, migrants in vulnerable situations or people who have been in prison. The team is multidisciplinary, consisting of people with lived experience working as peer advocates, nurse specialists, social and outreach workers, radiographers, and doctors with primary/secondary care expertise. They use state of the art point of care diagnostics within outreach vans to help overcome barriers in access to care among socially excluded populations.
3. ‘Gypsy & Traveller Exchange’ (Leeds gate, 2022): An example of a successful programme of raising awareness is this programme, led by community members and working in partnership with local services, supported by funding from charitable organisations and local government. Their strategy is to identify community members (or ‘gatekeepers’) who are trusted to facilitate communication with healthcare services, lead cultural awareness training for healthcare workers and provide community outreach and dedicated services for Roma and Traveller populations (McFadden et al., 2018; Condon et al., 2019; Carr et al., 2014). Similar approaches could be successful in other populations to provide education and support.
4. ‘One Stop Liver Shop’ (Hla et al., 2020): The Aboriginal population in the Northern Territory has the highest prevalence of HBV in Australia (6.08% compared to the Non-Indigenous population 1.56%). The ‘One Stop Liver Shop’ was developed iteratively with a very remote Aboriginal community (>500 km from the nearest tertiary hospital in Darwin). It is made up of a specialist doctor, community based Aboriginal Health Practitioner, a sonographer and clinical nurse specialist. They use a portable ultrasound scan, a FibroSan for transient elastography and a mobile device to use the ‘Hep B Story App’, a mobile application using the relevant local language. Visits occur 4 times per year. Due to this initiative, 88% of those aware of their diagnosis were engaged in care and 16% were on treatment.
All pregnant women should be screened for HBV, and healthcare professionals should consider opportunistically offering HBV screening in high-risk groups accessing services (World Health Organization, 2020; Centers for Disease Control and Prevention, 2022). There should also be a move to integrate routine hepatitis screening into high-risk healthcare settings, for example TB, sexual health and HIV services. The European Centre for Disease Prevention and Control (ECDC) recommends universal screening in emergency departments in areas where HBV prevalence exceeds 2%, however, evidence suggests that universal emergency department screening is cost effective even at lower prevalence (>0.25%) and therefore the United Kingdom is currently implementing this policy (Williams et al., 2020b, Nebbia et al., 2022).
Multiple studies have demonstrated cost-effectiveness of HBV screening in a variety of different scenarios, including community-based outreach and low prevalence settings (Su et al., 2022, Xiao et al., 2020, Toy et al., 2022, Nayagam et al., 2016, Tordrup et al., 2020, Hutton et al., 2022, Wright et al., 2018). This is largely due to averting high costs associated with decompensated cirrhosis, HCC and liver transplantation.
Rethinking prevention strategies
A safe, effective vaccine against HBV has been available since 1982, and remains a key tool for the elimination of viral hepatitis (World Health Organization, 2017). The WHO has recommended a universal birth dose HBV vaccination since 2009, irrespective of the mother’s serological status, but coverage was still only 42% globally and 17% in the WHO African Region by 2021 (World Health Organization, 2022a). Modelling suggests that scaling up of a timely birth dose vaccine to >90% by 2030 would result in immediate reduction of chronic HBV incidence, and 710,000 fewer deaths in those born between 2020–2030, globally (de Villiers et al., 2021). This strategy would be a particularly important public health intervention for future inclusion health populations, in whom catch-up three-dose adult vaccination is challenging and may not be cost-effective.
While catch-up adult vaccination is not advocated at a general population level, opportunities for providing vaccination to high-risk adults must still be explored and developed, optimising approaches to deliver a three-dose schedule alongside education, proactive antenatal and sexual healthcare, and other health interventions (Box 2).
Example methods of increasing HBV vaccination coverage among high-risk groups
Universal HBV vaccination in prisons in Scotland (Palmateer et al., 2018).
Following prison-related outbreaks of acute HBV among PWID, HBV vaccination for all prisoners was introduced in 1999.
Among recent-onset PWID in Glasgow, vaccine uptake increased from 16% in 1993 to 59% in 2009–2014 (p<0.001).
HBsAg prevalence 0.3% among people who commenced injecting drugs in the decade since universal vaccination induced, compared to 9% globally.
Vaccination is associated with 40% reduced odds of ever having HBV (aOR = 0.6, 95% CI 0.37–0.97).
HBV-COMSAVA (Community Screening and Vaccination in Africans) Study, Barcelona, Spain (Picchio et al., 2021).
Pop-up clinics at west African migrant faith-based and community organisations.
Individuals offered a rapid HBsAg diagnostic test and a dried blood sample for analysing HBV viral load and co-infection with hepatitis D virus.
If HBsAg +, same day referral to specialist with expedited referral process which did not require prior appointment.
If susceptible, offered HBV vaccine in situ at return appointment (‘return-date slip’ reminder given at first appointment).
70% were susceptible to HBV, 74% returned to receive results and 86% of those who returned accepted vaccination.
Sex-worker outreach, Ghent, Belgium (Mak et al., 2003)
A medical doctor and social nurse visited the workplaces of sex-workers, discussed professional risks, handed out prevention materials and offered free STI screening (Chlamydia, gonorrhoea, syphilis, HIV), a cervical smear and free HBV vaccination.
Psychological and legal programs related to sex work could also be discussed and anonymity guaranteed.
Over two-thirds of susceptible sex-workers completed an accelerated HBV vaccination schedule (over 2 months), compared to less than half if given over 6 months.
Only 7% were vaccinated in existing services.
Given the slow and heterogenous uptake of birth dose vaccination, and challenges associated with catch-up vaccination of inclusion health populations, other prevention strategies need urgent consideration. For example, the ‘Test and Treat’ strategy which aims to suppress the reservoir of virus in the population (McNaughton et al., 2019). According to current guidelines, only 25–35% of adults with CHB are eligible for treatment, leaving the majority at risk of both longer term complications (relevant to the individual) and the potential for transmission (relevant to populations) (McNaughton et al., 2021). HBV/HIV co-infected individuals may have a treatment advantage, reflecting the benefits of greater access to early and consistent antiviral treatment compared to mono-infected individuals (Maponga et al., 2020). Potential risks should be considered: toxicity and side effects, requirement for monitoring, potential for drug resistance, limited gains from treating well people and the cost of drug and potential diversion of healthcare resources (McNaughton et al., 2021). Further research is required to assess the efficacy, feasibility and acceptability of wider treatment roll out.
Designing inclusive HBV healthcare
Once diagnosed, HBV requires long-term surveillance. However, current models of healthcare fail to provide adequate continuity for inclusion health populations, and innovative solutions are needed. Interventions to reduce the burden of vaccine-preventable infections among migrants can be classified based on the individual, community, provider and system (Charania et al., 2020). This framework can be applied to all inclusion health populations (Box 2, Figure 2).
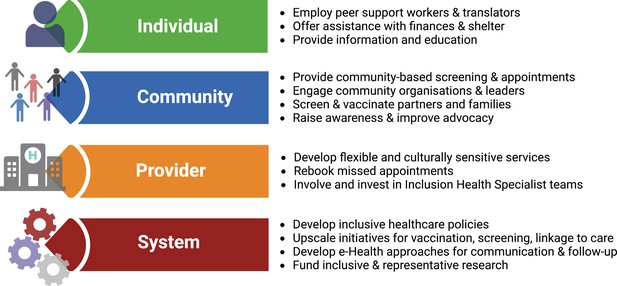
Solutions for service development to overcome barriers for people living with hepatitis B virus (HBV) infection in inclusion health populations, applying framework suggested by Charania et al., 2020.
Figure created in BioRender.com with a licence to publish.
Developing holistic approaches
Inclusion health populations face many challenges; therefore, the HBV agenda must not operate in a silo. Integrated infectious disease screening can be cost-effective and an efficient use of resources. An example is the World Health Organization’s ‘triple elimination initiative’ which encourages countries to simultaneously commit to elimination of mother-to-child transmission of HIV, syphilis and HBV (World Health Organization, 2022b). This approach has been demonstrated to reduce disease transmission rates, staff and patient time, and overall programme cost (Zhang et al., 2019).
Integrated infection screening is also recommended in migrants, and in most countries screening tests are offered following a risk assessment (European Centre for Disease Prevention and Control, 2018). Screening is found to be cost-effective, feasible and acceptable, but migrants are a heterogenous group, and services should be culturally appropriate, and accommodate the possibility of fear and stigma surrounding screening and diagnosis (Seedat et al., 2018; Cinardo et al., 2022). In many countries, migrants will undergo pre-entry or port-of-entry screening, missing those who arrive through informal routes (Cinardo et al., 2022). Culturally sensitive outreach services are an important way to reach and screen inclusion health populations (Box 1).
Multi-morbidity is common, for example, incarcerated individuals with HBV have high rates of HIV, HCV and /or TB co-infection (Ahmadi Gharaei et al., 2021; do Nascimento et al., 2020; La Torre et al., 2007), and of substance use and severe mental illness (Kamarulzaman et al., 2016; Fazel and Danesh, 2002). Non-communicable diseases are also prevalent among inclusion health populations (Tweed et al., 2021) for example, PEH have a three-fold elevated risk of cardiovascular disease and cardiovascular mortality compared to non-homeless individuals (Table 2; Al Shakarchi et al., 2020). Moreover, people with co-existing exposures, for example incarceration and substance use, or serious mental illness and substance use, have worse outcomes (Tweed et al., 2021). Diabetes is associated with an increased risk of HBV-associated HCC (hazard ratio 1.36, 95% CI 1.23–1.49)(Campbell et al., 2021). The HBV inclusion health agenda must therefore take a ‘syndemic’ approach, namely to tackle viral hepatitis not as an isolated challenge but recognising its place amongst complex social, physical and mental health challenges and addressing the person as a whole (Mendenhall, 2017; Matthews et al., 2022b).
Prioritising patient and public involvement
HBV infection can result in stigma and discrimination, which is magnified when combined with the experiences of socially excluded groups (Matthews et al., 2022a). Sharing lived experiences builds rapport, trust and equality, helping promote advocacy, build peer support networks, navigate healthcare systems and promote diagnosis and treatment of blood borne infections (Paisi et al., 2022; Crone et al., 2022). There is a long history of peer-led approaches with successful outcomes in HIV, TB and HCV (Story et al., 2020; Øgård-Repål et al., 2023). For example, as part of the HepCare project, people with lived experience of HCV infection were upskilled to become equal members of the healthcare team, providing a cost-effective approach to enhancing diagnosis, linkage to care and treatment success (Ward et al., 2019; Surey et al., 2021; Surey et al., 2019). Importantly, a focus group involving people with lived experience ranked advocacy via peer-led services as the second most important intervention behind stable housing (Luchenski et al., 2018). The success and knowledge gained from this peer-led approach must now be translated to HBV.
Improving awareness, education, and advocacy
Qualitative research from Australia has identified lack of confidence of healthcare professionals and poor communication surrounding HBV (Wallace et al., 2011; Robotin et al., 2021). Sensitive communication around HBV is of heightened importance in populations who already experience discrimination and stigma, and is a particular consideration where certain subjects are culturally taboo (Condon et al., 2019). Engagement requires sensitivity to cultural nuances, including traditional and religious beliefs (Holmstrom, 2019, Carr et al., 2014). Training for healthcare professionals in trauma-informed care would benefit many inclusion health populations who are more likely to have a history of experiencing assault and torture (Emily, 2021). Lack of awareness is present in healthcare professionals as well as in at-risk populations (Cochrane et al., 2016; Bierhoff et al., 2021). A UK survey of primary care practitioners found many did not believe routine testing of HBV in migrants was necessary, and national guidelines were poorly followed (Evlampidou et al., 2016). Interventions to raise HBV awareness in different groups are paramount to inform action for inclusion health populations.
Representative research
Inclusion health populations are often seen as ‘hard to reach’ and may not be viewed as ideal research participants given the possibility of increased loss to follow up and co-morbid conditions. This is further exacerbated in low-resource settings, where there is higher HBV prevalence. There is a striking lack of inclusion health research within Africa, even among global reviews (Table 2). In order to be generalisable and equitable, HBV research needs to be representative of real-world populations and challenges, on a global scale. Performance of new biomarkers, for example hepatitis B core-related antigen, or clinical fibrosis scores, such as APRI and FIB-4, needs to be validated for clinical utility in these populations with overlapping risk factors, multi-morbidity and in varying global populations. While limited treatments currently exist for HBV infection, there is active research into improved treatment and curative therapies; for these to be relevant in populations most affected by HBV, clinical trials must recruit accordingly. Mixed-method approaches should be utilised to understand views and unmet needs of marginalised communities in relation to HBV. The exclusion of inclusion health populations from the current research agenda is likely to further exacerbate inequities when more effective treatments do become available.
Conclusion
Inclusion health populations comprise diverse groups, but share common experiences including stigma, discrimination, marginalisation, and barriers to accessing diagnostic services and engaging with long-term care. There is a disproportionate impact of HBV among these populations, both caused and exacerbated by social exclusion, which is associated with increased morbidity and mortality. These challenges are even greater in resource-limited settings, where HBV burden is higher but robust data are lacking.
The power and impact of existing studies representing HBV in these populations are limited by small size, cross sectional design and heterogeneity. There are few investigations into interventions, scarce qualitative data, and limited representation of people with lived experience. Moreover, patient advocacy groups for HBV, particularly in inclusion health populations, do not have the same presence or traction as for other infectious diseases, for example TB or HIV. More well-designed prospective studies are needed to allow investigation of the complex interplay between social adversity, co-morbidity and HBV infection to better understand its epidemiology, risk factors, pathogenesis and outcomes within under-served populations. Representative research is crucial to ensure that new discoveries, for example treatments, biomarkers and risk scores, are equitable, relevant, applicable and accessible to the populations where they are most relevant, while qualitative research is needed to understand the perspectives and priorities of the HBV community and inform more effective messaging and advocacy. Innovative methods are urgently required to overcome barriers, adapt healthcare and create services that are fit-for-purpose for inclusion health populations, integrating HBV care into holistic strategies that provide interdisciplinary care to address the complex overlapping needs of inclusion health populations. HBV epitomises the need to adopt a syndemic approach that recognises and addresses the complex interplay between comorbidity and sociocultural contexts.
References
-
Alcohol and HIV sexual risk behaviors among injection drug usersDrug and Alcohol Dependence 95:54–61.https://doi.org/10.1016/j.drugalcdep.2007.12.008
-
Description and evaluation of a pathway for unaccompanied asylum-seeking childrenArchives of Disease in Childhood 107:456–460.https://doi.org/10.1136/archdischild-2021-322319
-
Health-seeking behaviours in the homeless population: a concept analysisHealth & Social Care in the Community 30:e278–e286.https://doi.org/10.1111/hsc.13499
-
Release from prison -- a high risk of death for former inmatesThe New England Journal of Medicine 356:157–165.https://doi.org/10.1056/NEJMsa064115
-
WebsiteAs Cities Grow, So Do the Numbers of HomelessYale University Macmillan Center. Accessed August 22, 2020.
-
Screening for neglected tropical diseases and other infections in refugee and asylum-seeker populations in the united kingdomTherapeutic Advances in Infectious Disease 9:20499361221116680.https://doi.org/10.1177/20499361221116680
-
Barriers and opportunities for hepatitis B testing and contact tracing in a UK somali population: a qualitative studyEuropean Journal of Public Health 26:389–395.https://doi.org/10.1093/eurpub/ckv236
-
Italian migrants study: an HCV and HBV micro-elimination pilot projectClinics and Research in Hepatology and Gastroenterology 46:101852.https://doi.org/10.1016/j.clinre.2021.101852
-
Engaging gypsy, roma, and traveller communities in research: maximizing opportunities and overcoming challengesQualitative Health Research 29:1324–1333.https://doi.org/10.1177/1049732318813558
-
Accelerating the elimination of viral hepatitis: a lancet gastroenterology & hepatology commissionThe Lancet. Gastroenterology & Hepatology 4:135–184.https://doi.org/10.1016/S2468-1253(18)30270-X
-
ReportCouncil of Europe Strategic Action Plan for Roma and Traveller Inclusion (2020 - 2025)Council of Europe.
-
Health service access among homeless veterans: health access challenges faced by homeless African American veteransJournal of Racial and Ethnic Health Disparities 9:1828–1844.https://doi.org/10.1007/s40615-021-01119-z
-
Risk prison and hepatitis B virus infection among inmates with history of drug injection in isfahan, iranTheScientificWorldJournal 2013:735761.https://doi.org/10.1155/2013/735761
-
Towards genotype-specific care for chronic hepatitis B: the first 6 years follow up from the CHARM cohort studyOpen Forum Infectious Diseases 6:fz469.https://doi.org/10.1093/ofid/ofz469
-
First report on sexually transmitted infections among trans (male to female transvestites, transsexuals, or transgender) and male sex workers in argentina: high HIV, HPV, HBV, and syphilis prevalenceInternational Journal of Infectious Diseases 15:e635–e640.https://doi.org/10.1016/j.ijid.2011.05.007
-
BookClinics for migrant and refugee children: psychosocial and organizational considerationsIn: Esmaili E, editors. Child Refugee and Migrant Health: A Manual for Health Professionals. Switzerland: Springer Nature. pp. 87–96.https://doi.org/10.1007/978-3-030-74906-4_10
-
ReportEpidemiological assessment of hepatitis B and C among migrants in the EU/EEAStockholm.
-
Low hepatitis B testing among migrants: a cross-sectional study in a UK cityThe British Journal of General Practice 66:e382–e391.https://doi.org/10.3399/bjgp16X684817
-
Health-related quality of life for adults living with hepatitis B in the united states: a qualitative assessmentJournal of Patient-Reported Outcomes 5:121.https://doi.org/10.1186/s41687-021-00398-8
-
Incarceration and injection drug use in baltimore, marylandAddiction 110:1152–1159.https://doi.org/10.1111/add.12938
-
Alcohol and viral hepatitis: a mini-reviewDigestive and Liver Disease 41:67–70.https://doi.org/10.1016/j.dld.2008.05.009
-
Sex and condom use in a large jail unit for men who have sex with men (MSM) and male-to-female transgendersJournal of Health Care for the Poor and Underserved 21:1071–1087.https://doi.org/10.1353/hpu.0.0349
-
A “one stop liver shop” approach improves the cascade-of-care for aboriginal and torres strait islander australians living with chronic hepatitis B in the northern territory of australia: results of A novel care delivery modelInternational Journal for Equity in Health 19:64.https://doi.org/10.1186/s12939-020-01180-w
-
Cost-Effectiveness of hepatitis B testing and vaccination of adults seeking care for sexually transmitted infectionsSexually Transmitted Diseases 49:517–525.https://doi.org/10.1097/OLQ.0000000000001632
-
Self-Reported experiences of health services among female street-based prostitutes: a cross-sectional surveyThe British Journal of General Practice 54:515–519.
-
Improving care of migrants is key for viral hepatitis elimination in EuropeBulletin of the World Health Organization 99:280–286.https://doi.org/10.2471/BLT.20.260919
-
Male sex workers in Antwerp, Belgium: a descriptive studyInternational Journal of STD & AIDS 16:744–748.https://doi.org/10.1258/095646205774763072
-
Alcohol and HBV synergistically promote hepatic steatosisAnnals of Hepatology 18:913–917.https://doi.org/10.1016/j.aohep.2019.04.013
-
The Roma population living in segregated settlements in eastern Slovakia has a higher prevalence of metabolic syndrome, kidney disease, viral hepatitis B and E, and some parasitic diseases compared to the majority populationInternational Journal of Environmental Research and Public Health 17:3112.https://doi.org/10.3390/ijerph17093112
-
Meta-analysis of the prevalence of HBV infection among alcohol users worldwideAlcohol and Alcoholism 55:136–143.https://doi.org/10.1093/alcalc/agz102
-
Hepatitis B vaccination for sex workers: do outreach programmes perform better?Sexually Transmitted Infections 79:157–159.https://doi.org/10.1136/sti.79.2.157
-
Treatment advantage in HBV/HIV coinfection compared to HBV monoinfection in a South African cohortThe Journal of Infection 81:121–130.https://doi.org/10.1016/j.jinf.2020.04.037
-
A call for advocacy and patient voice to eliminate hepatitis B virus infectionThe Lancet. Gastroenterology & Hepatology 7:282–285.https://doi.org/10.1016/S2468-1253(21)00475-1
-
Outcome of HBV screening and vaccination in a migrant population in southern ItalyLe Infezioni in Medicina 29:236–241.
-
Gypsy, Roma and traveller access to and engagement with health services: a systematic reviewEuropean Journal of Public Health 28:74–81.https://doi.org/10.1093/eurpub/ckx226
-
Extending treatment eligibility for chronic hepatitis B virus infectionNature Reviews. Gastroenterology & Hepatology 18:146–147.https://doi.org/10.1038/s41575-020-00398-x
-
Seroprevalence of HBV and HCV in female sex workers from four cities in the state of pará, Northern BrazilJournal of Medical Virology 93:3730–3737.https://doi.org/10.1002/jmv.26759
-
ConferenceBarriers to recruitment of adults with HBV for participation in clinical research studiesConference on Liver Disease in Africa. Virtual.
-
“Health is on the back burner:” multilevel barriers and facilitators to primary care among people who inject drugsJournal of General Internal Medicine 36:129–137.https://doi.org/10.1007/s11606-020-06201-6
-
Hepatitis B virus infection in EU/EEA and united kingdom prisons: a descriptive analysisEpidemiology and Infection 149:e59.https://doi.org/10.1017/S0950268821000169
-
WebsiteEstimates of Funding for Various Research, Condition, and Disease CategoriesAccessed November 24, 2022.
-
A review of a GP registrar-run mobile health clinic for homeless peopleIrish Journal of Medical Science 186:541–546.https://doi.org/10.1007/s11845-016-1527-2
-
Peer support for people living with HIV: A scoping reviewHealth Promotion Practice 24:172–190.https://doi.org/10.1177/15248399211049824
-
Hepatitis B virus infection as a neglected tropical diseasePLOS Neglected Tropical Diseases 11:e0005842.https://doi.org/10.1371/journal.pntd.0005842
-
Injecting drug use in low and middle-income countries: opportunities to improve care and prevent harmJournal of Viral Hepatitis 24:714–724.https://doi.org/10.1111/jvh.12741
-
Effectiveness of a screening program for HBV, HCV, and HIV infections in african migrants to sicilyDigestive and Liver Disease 54:800–804.https://doi.org/10.1016/j.dld.2021.08.024
-
Hepatitis B in the Northern Territory: insights into the changing epidemiology of an ancient conditionInternal Medicine Journal 51:910–922.https://doi.org/10.1111/imj.15069
-
Community-Based hepatitis B screening: what works?Hepatology International 8:478–492.https://doi.org/10.1007/s12072-014-9562-4
-
Hepatitis B and liver cancer: community awareness, knowledge and beliefs of middle eastern migrants in sydney, australiaInternational Journal of Environmental Research and Public Health 18:8534.https://doi.org/10.3390/ijerph18168534
-
HIV, syphilis, and viral hepatitis among latin american indigenous peoples and afro-descendants: a systematic reviewRevista Panamericana de Salud Publica = Pan American Journal of Public Health 43:e17.https://doi.org/10.26633/RPSP.2019.17
-
Hepatitis B, hepatitis C and HIV prevalence and related sexual and substance use risk practices among key populations who access HIV prevention, treatment and related services in south africa: findings from a seven-city cross-sectional survey (2017)BMC Infectious Diseases 20:655.https://doi.org/10.1186/s12879-020-05359-y
-
Hiv, hepatitis B and C, and syphilis prevalence and coinfection among sex workers in southern brazilRevista Da Sociedade Brasileira de Medicina Tropical 46:493–497.https://doi.org/10.1590/0037-8682-1364-2013
-
How effective are approaches to migrant screening for infectious diseases in Europe? A systematic reviewThe Lancet. Infectious Diseases 18:e259–e271.https://doi.org/10.1016/S1473-3099(18)30117-8
-
ReportVA evidence-based synthesis program reports Primary Care Engagement Among Veterans with Experiences of Homelessness and Serious Mental Illness: An Evidence MapDepartment of Veterans Affairs.
-
Management and control of tuberculosis control in socially complex groups: a research programme including three rctsProgramme Grants for Applied Research 8:1–76.https://doi.org/10.3310/pgfar08090
-
From peer-based to peer-led: redefining the role of peers across the hepatitis C care pathway: hepcare EuropeThe Journal of Antimicrobial Chemotherapy 74:v17–v23.https://doi.org/10.1093/jac/dkz452
-
Cost-Effectiveness of 1-time universal screening for chronic hepatitis B infection in adults in the United StatesClinical Infectious Diseases 74:210–217.https://doi.org/10.1093/cid/ciab405
-
Health of people experiencing co-occurring homelessness, imprisonment, substance use, sex work and/or severe mental illness in high-income countries: a systematic review and meta-analysisJournal of Epidemiology and Community Health 75:1010–1018.https://doi.org/10.1136/jech-2020-215975
-
ReportKnowledge, attitude and practices of commercial sex workers towards hepatitis B: understanding the gapsWebversion Impact Report.
-
High hepatitis B and low hepatitis C prevalence in roma population in eastern slovakiaCentral European Journal of Public Health 22:S51–S56.https://doi.org/10.21101/cejph.a3902
-
The cost-effectiveness of an HCV outreach intervention for at-risk populations in london, UKThe Journal of Antimicrobial Chemotherapy 74:v5–v16.https://doi.org/10.1093/jac/dkz451
-
Screening for infection in unaccompanied asylum-seeking children and young peopleArchives of Disease in Childhood 105:530–532.https://doi.org/10.1136/archdischild-2019-318077
-
Hiv and viral hepatitis among imprisoned key populationsEpidemiologic Reviews 40:12–26.https://doi.org/10.1093/epirev/mxy003
-
ReportGlobal Status Report on Alcohol and Health GenevaSwitzerland: World Health Organisation.
-
ReportReport on the Health of Refugees and Migrants in the WHO European RegionGeneva, Switzerland: World Health Organisation.
-
Enhancing the hepatitis B care cascade in Australia: a cost-effectiveness modelJournal of Viral Hepatitis 27:526–536.https://doi.org/10.1111/jvh.13252
-
Treatment gap, help-seeking, stigma and magnitude of alcohol use disorder in rural ethiopiaSubstance Abuse Treatment, Prevention, and Policy 14:4.https://doi.org/10.1186/s13011-019-0192-7
Article and author information
Author details
Funding
Wellcome Trust (110110)
- Philippa C Matthews
Francis Crick Institute
- Philippa C Matthews
University College London Hospitals Biomedical Research Centre
- Philippa C Matthews
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. For the purpose of Open Access, the authors have applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.
Acknowledgements
PCM is funded by Wellcome (grant ref 110110/Z/15/Z), UCLH NIHR Biomedical Research Centre, and the Francis Crick Institute. AS is Clinical Lead for UCLH Find&Treat Service and UCL Professor of Inclusion Health. EM is an NIHR funded academic clinical fellow. BS receives doctoral funding from NIHR.
Copyright
© 2023, Martyn et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 5,975
- views
-
- 356
- downloads
-
- 32
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Epidemiology and Global Health
- Genetics and Genomics
Alzheimer’s disease (AD) is a complex degenerative disease of the central nervous system, and elucidating its pathogenesis remains challenging. In this study, we used the inverse-variance weighted (IVW) model as the major analysis method to perform hypothesis-free Mendelian randomization (MR) analysis on the data from MRC IEU OpenGWAS (18,097 exposure traits and 16 AD outcome traits), and conducted sensitivity analysis with six models, to assess the robustness of the IVW results, to identify various classes of risk or protective factors for AD, early-onset AD, and late-onset AD. We generated 400,274 data entries in total, among which the major analysis method of the IVW model consists of 73,129 records with 4840 exposure traits, which fall into 10 categories: Disease, Medical laboratory science, Imaging, Anthropometric, Treatment, Molecular trait, Gut microbiota, Past history, Family history, and Lifestyle trait. More importantly, a freely accessed online platform called MRAD (https://gwasmrad.com/mrad/) has been developed using the Shiny package with MR analysis results. Additionally, novel potential AD therapeutic targets (CD33, TBCA, VPS29, GNAI3, PSME1) are identified, among which CD33 was positively associated with the main outcome traits of AD, as well as with both EOAD and LOAD. TBCA and VPS29 were negatively associated with the main outcome traits of AD, as well as with both EOAD and LOAD. GNAI3 and PSME1 were negatively associated with the main outcome traits of AD, as well as with LOAD, but had no significant causal association with EOAD. The findings of our research advance our understanding of the etiology of AD.
-
- Epidemiology and Global Health
Artificially sweetened beverages containing noncaloric monosaccharides were suggested as healthier alternatives to sugar-sweetened beverages. Nevertheless, the potential detrimental effects of these noncaloric monosaccharides on blood vessel function remain inadequately understood. We have established a zebrafish model that exhibits significant excessive angiogenesis induced by high glucose, resembling the hyperangiogenic characteristics observed in proliferative diabetic retinopathy (PDR). Utilizing this model, we observed that glucose and noncaloric monosaccharides could induce excessive formation of blood vessels, especially intersegmental vessels (ISVs). The excessively branched vessels were observed to be formed by ectopic activation of quiescent endothelial cells (ECs) into tip cells. Single-cell transcriptomic sequencing analysis of the ECs in the embryos exposed to high glucose revealed an augmented ratio of capillary ECs, proliferating ECs, and a series of upregulated proangiogenic genes. Further analysis and experiments validated that reduced foxo1a mediated the excessive angiogenesis induced by monosaccharides via upregulating the expression of marcksl1a. This study has provided new evidence showing the negative effects of noncaloric monosaccharides on the vascular system and the underlying mechanisms.






