Allorecognition: Knowing friend from foe
Many multicellular organisms have mechanisms in place that allow them to detect which cells belong to them, and which cells are from another organism. Being able to discriminate self from non-self, known as allorecognition, is vital for the sustainability of life. For instance, our immune system uses this mechanism to identify and attack non-self cells and tissues, which is why our bodies sometimes reject transplanted organs donated from someone else (Callemeyn et al., 2022).
Filamentous fungi – which are made up of microscopic thread-like structures called hypha – also rely on allorecognition to decide what to do when they come across hyphae from other fungi. Merging with other hyphae would allow the fungus to expand its network and access more resources that may benefit its survival. However, before this can happen, the fungus uses allorecognition to determine if a newly encountered hypha is safe to fuse to.
In the fungus Neurospora crassa, this process of allorecognition involves three checkpoints (Gonçalves et al., 2020; Zhao et al., 2015). First, the hyphae release chemical signals that attract fusion compatible hyphae (Heller et al., 2016). Second, two proteins called CWR-1 and CWR-2 determine whether the cell wall surrounding the hyphae will dissolve so the cells can merge their membranes and mix their cytoplasmic content (Gonçalves et al., 2019). Once the fungi fuse, final checks are carried out, with the failure of these tests triggering the death of the newly joined hypha. Now, in eLife, Louise Glass and co-workers from the University of California, Berkeley – including Tyler Detomasi and Adriana Rico-Ramírez as joint first authors – report new insights into how the CWR-1 protein regulates the second checkpoint of allorecognition (Detomasi et al., 2022).
N. crassa have different versions, or alleles, of the genes that encode CWR-1 and CWR-2, and these can be divided in to six different ‘haplogroups’ based on their degree of similarity (Gonçalves et al., 2019). Only N. crassa with CWR-1 and CWR-2 proteins from the same haplogroup can fuse: if the gene for CWR-1 in one hypha is in a different haplogroup to the gene for CWR-2 in the other, their cells walls will remain intact and their membranes will not merge (Figure 1). This shows that variations in these two proteins determine whether or not hyphae are compatible for fusion.
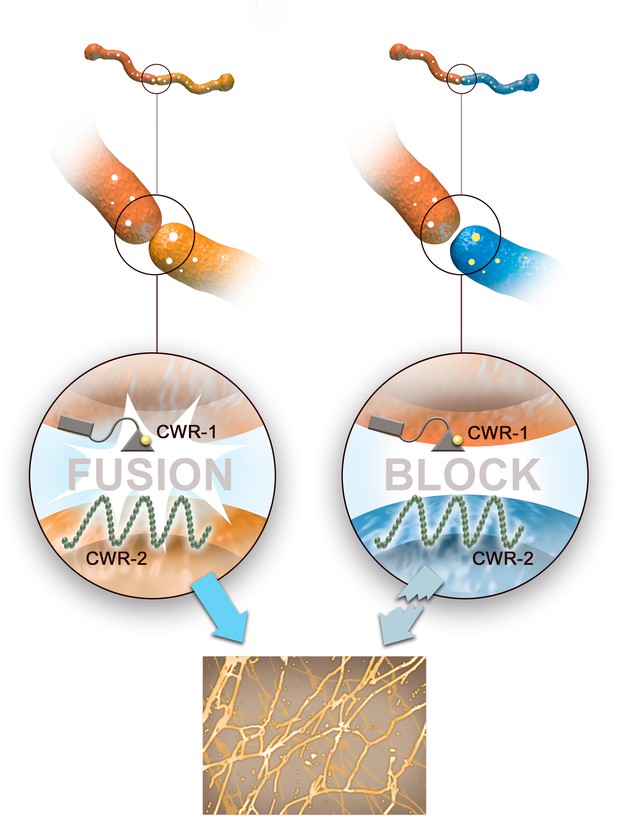
The second checkpoint of allorecognition in filamentous fungi.
When hyphae from two distinct fungi come into contact, a series of checkpoints are initiated to make sure the fungi are genetically compatible. The second stage of this allorecognition process is regulated by two proteins at the cell wall called CWR-1 and CWR-2. If the two hyphae contain CWR-1 and CWR-2 proteins from the same haplogroup (left), the hyphae dissolve their cells walls, merge their membranes, and mix their cytoplasmic content together. This allows the fungi to expand their network of interconnected hyphae (bottom panel). If the two hyphae contain CWR-1 and CWR-2 proteins from different haplogroups (right), the cell wall does not dissolve and the genetically incompatible hyphae cannot proceed with fusion.
Image credit: Henning Dalhoff.
Using well-established methods, Detomasi et al. revealed that the CWR-1 protein is part of a family of copper-containing enzymes called lytic polysaccharide monooxygenases, or LPMOs for short. Similar to LPMOs found in other fungal species, the CWR-1 proteins from all six haplogroups degrade the polysaccharide chitin, a long-chain carbohydrate that maintains the structure of the cell wall and helps anchor other cell wall components in place (Brown et al., 2019). This enzymatic activity depends on the copper in the protein, which is coordinated by two amino acids in what is known known as a histidine brace (Ipsen et al., 2021). Surprisingly, Detomasi et al. found that mutating the histidine brace of CWR-1 did not stop N. crassa strains from exhibiting normal allorecognition and only fusing with genetically compatible fungi, despite the enzyme being inactive.
Through a series of clever genetic mutations, Detomasi et al. found that CWR-1 does not need its enzymatic activity or the domains of the protein that bind to chitin to carry out its role in allorecognition. It does, however, require its catalytic domain. Further mutations showed that modifying regions in the catalytic domain of CWR-1 that are predicted to interact with chitin (but are not responsible for the protein’s enzymatic activity) altered which N. crassa strains could fuse their hyphae together. This suggests that these sections of the CWR-1 protein confer the allele specificity needed for cells to pass the second checkpoint of allorecognition.
To our knowledge, this is the first time a LPMO protein has been shown to have a function that does not involve the degradation of polysaccharides. However, LPMO-like proteins which do not catalyze the breakdown of carbohydrates have been found in other fungal species (Garcia-Santamarina et al., 2020; Labourel et al., 2020). While these proteins look like LMPOs based on their amino acid sequence, a closer inspection reveals that their copper-binding sites are slightly different than expected. These LPMO-like proteins have been shown to be important for copper import in the fungal species Cryptococcus neoformans, and for establishing a symbiosis relationship between the fungus Laccaria bicolor and plant roots.
This work is a major step towards understanding allorecognition in fungi, but several questions remain. As Detomasi et al. point out, future work is needed to probe how CWR-1 and CWR-2 mechanically block cell fusion. Furthermore, it is still unclear if and how CWR-1, which binds to chitin in the cell wall, gets in to contact with the CWR-2 protein on the membrane of the neighboring hypha despite there being two layers of cell wall between them. Finally, while initial investigations suggest that the CWR-1/CWR-2 model likely occurs in other species (Gonçalves et al., 2019), it is still uncertain how widespread this mechanism is across the fungal kingdom.
References
-
BookChitin: a “hidden figure” in the fungal cell wallIn: Latgé JP, editors. The Fungal Cell Wall. Springer. pp. 83–111.https://doi.org/10.1007/82_2019_184
-
Allorecognition and the spectrum of kidney transplant rejectionKidney International 101:692–710.https://doi.org/10.1016/j.kint.2021.11.029
-
Conflict, competition, and cooperation regulate social interactions in filamentous fungiAnnual Review of Microbiology 74:693–712.https://doi.org/10.1146/annurev-micro-012420-080905
-
A fungal family of lytic polysaccharide monooxygenase-like copper proteinsNature Chemical Biology 16:345–350.https://doi.org/10.1038/s41589-019-0438-8
-
Identification of allorecognition loci in Neurospora crassa by genomics and evolutionary approachesMolecular Biology and Evolution 32:2417–2432.https://doi.org/10.1093/molbev/msv125
Article and author information
Author details
Publication history
Copyright
© 2022, Hallas-Møller and Johansen
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 527
- views
-
- 89
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Chromosomes and Gene Expression
- Genetics and Genomics
Among the major classes of RNAs in the cell, tRNAs remain the most difficult to characterize via deep sequencing approaches, as tRNA structure and nucleotide modifications can each interfere with cDNA synthesis by commonly-used reverse transcriptases (RTs). Here, we benchmark a recently-developed RNA cloning protocol, termed Ordered Two-Template Relay (OTTR), to characterize intact tRNAs and tRNA fragments in budding yeast and in mouse tissues. We show that OTTR successfully captures both full-length tRNAs and tRNA fragments in budding yeast and in mouse reproductive tissues without any prior enzymatic treatment, and that tRNA cloning efficiency can be further enhanced via AlkB-mediated demethylation of modified nucleotides. As with other recent tRNA cloning protocols, we find that a subset of nucleotide modifications leave misincorporation signatures in OTTR datasets, enabling their detection without any additional protocol steps. Focusing on tRNA cleavage products, we compare OTTR with several standard small RNA-Seq protocols, finding that OTTR provides the most accurate picture of tRNA fragment levels by comparison to "ground truth" Northern blots. Applying this protocol to mature mouse spermatozoa, our data dramatically alter our understanding of the small RNA cargo of mature mammalian sperm, revealing a far more complex population of tRNA fragments - including both 5′ and 3′ tRNA halves derived from the majority of tRNAs – than previously appreciated. Taken together, our data confirm the superior performance of OTTR to commercial protocols in analysis of tRNA fragments, and force a reappraisal of potential epigenetic functions of the sperm small RNA payload.
-
- Chromosomes and Gene Expression
- Genetics and Genomics
Models of nuclear genome organization often propose a binary division into active versus inactive compartments yet typically overlook nuclear bodies. Here, we integrated analysis of sequencing and image-based data to compare genome organization in four human cell types relative to three different nuclear locales: the nuclear lamina, nuclear speckles, and nucleoli. Although gene expression correlates mostly with nuclear speckle proximity, DNA replication timing correlates with proximity to multiple nuclear locales. Speckle attachment regions emerge as DNA replication initiation zones whose replication timing and gene composition vary with their attachment frequency. Most facultative LADs retain a partially repressed state as iLADs, despite their positioning in the nuclear interior. Knock out of two lamina proteins, Lamin A and LBR, causes a shift of H3K9me3-enriched LADs from lamina to nucleolus, and a reciprocal relocation of H3K27me3-enriched partially repressed iLADs from nucleolus to lamina. Thus, these partially repressed iLADs appear to compete with LADs for nuclear lamina attachment with consequences for replication timing. The nuclear organization in adherent cells is polarized with nuclear bodies and genomic regions segregating both radially and relative to the equatorial plane. Together, our results underscore the importance of considering genome organization relative to nuclear locales for a more complete understanding of the spatial and functional organization of the human genome.






