Neurotransmission: Unmasking a two-faced protein
To help you process this article, billions of neurons in your brain have been learning for years how to convert images and letters on the screen into words and ideas. The biochemical background for learning and memory is neuroplasticity: neurons emerge and die, form new synapses, and abandon old ones. The strength of each connection is fine-tuned by protein molecules communicating with each other inside a synapse.
One of the most abundant proteins in the postsynaptic density – the area of the neuron where nerve signals are amplified or repressed – is a protein called postsynaptic density 95 (PSD-95). This protein modulates interactions between hundreds of other proteins (Figure 1A; Wang et al., 2010; Fernández et al., 2009), and its structure and function change depending on synaptic activity (Bissen et al., 2019). Could it be that the different poses, or conformations, of the domains within PSD-95 specify which partners this protein interacts with inside the neuron, and hence allow neuroplasticity?
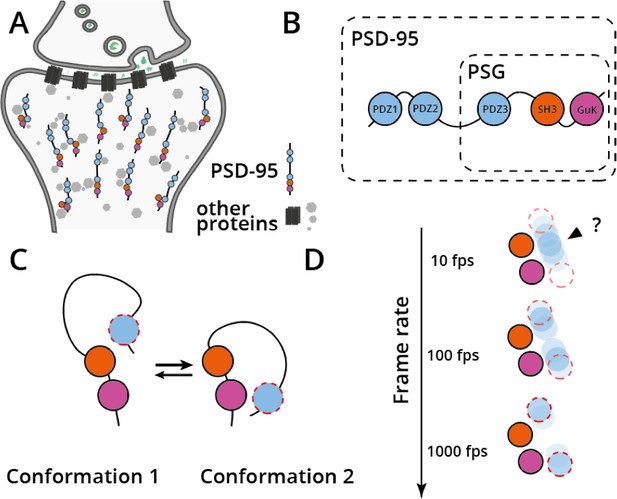
Synaptic localization, structure and dynamics of protein PSD-95.
(A) Protein PSD-95 (shown as five circles of different colors on a line) mediates protein-protein interactions in the postsynaptic density (other proteins are shown as grey circles or black squares) and hence modulates signal transduction in neurons (neurotransmitters are shown in green being released from the pre-synaptic cell). (B) Domain structure of PSD-95, showing the five domains of the protein (with the PDZ domains shown in light blue, the SH3 domain shown in orange and the GuK domain shown in pink). The PSG supramodule is indicated. (C) The PSG supramodule switches quickly between two conformations in which the PDZ3 domain (shown in light blue with a dashed red border) takes different positions. (D) High-speed recording of structural data overcomes averaging of distinct protein conformations. Top: when observed at 10 frames per second (fps), the positions of the SH3 and GuK domains (which remain still) are correctly resolved, but the PDZ3 domain is switching between its positions in the two conformations of the PSG supramodule faster than the images are being taken. This results in an ‘average’ conformation being recorded (shown in blue) instead of the two ‘real’ conformations with the correct positions of the PDZ3 domain (red dashed circles). Center: when observed at 100 fps, a similar thing happens, but in this case two (incorrect) averaged conformations are observed. Bottom: 1000 fps is a high enough frame rate to correctly resolve the two conformations of the PSG supramodule. Observed averaged conformations are shown in blue and marked with an arrowhead; true underlying conformations are shown as red dashed circles.
Now, in eLife, a group led by Mark Bowen (from Stony Brook University), Feng Ding and Hugo Sanabria (both from Clemson University) – with George Hamilton, Nabanita Saikia and Sujit Basak as joint first authors – reports on a structural model of PSD-95 based on single-molecule fluorescence microscopy and computer simulations. The group also confirms the predictions of the proposed model with a complimentary biochemical technique called disulfide screening, and reveal how ligand binding by one protein domain within PSD-95 is assisted by the proximity of another domain (Hamilton et al., 2022).
PSD-95 consists of five domains: three PDZ domains (PDZ1, PDZ2, PDZ3), an SH3 domain, and a GuK domain (Figure 1B). Three of these domains – PDZ3, SH3, and GuK – associate closely with each other and form a conserved supramodule called PSG. The two other domains, PDZ1 and PDZ2, are separated from PSG by a long flexible linker, and mostly interact with each other rather than with the other domains (McCann et al., 2012; McCann et al., 2011). Within PSG, the interaction between domains SH3 and GuK is tight, while the PDZ3 domain can ‘wiggle’ around them (Korkin et al., 2006; Tavares et al., 2001). In computer simulations, the PDZ3 domain can reach both its closest neighbor, the SH3 domain, and the more distant GuK domain, but it remained unclear whether both or only one of the two PDZ3 conformations occur in nature (Figure 1C; Korkin et al., 2006).
Hamilton et al. used single-molecule fluorescence microscopy to track the conformational dynamics of PSG within full-length PSD-95, showing that the PDZ3 domain switches between two major conformations with respect to the SH3-GuK complex (Figure 1C). In particular, they used a technique called single-molecule Förster resonance energy transfer (smFRET) – frequently referred to as a ‘molecular ruler’ – to measure the distances between the domains in PSG. Combining these measurements with a simulation technique called ‘rigid-body docking’ revealed the atomic structures of both conformations.
The conformations observed via smFRET are in good agreement with those that Hamilton et al. describe using discrete molecular dynamics simulations. In one conformation, the PDZ3 domain is close to the SH3 domain without contacting the GuK domain, while in the other, the PDZ3 domain is bound to the GuK domain. To validate the proposed atomic structures Hamilton et al. used disulfide screening. This technique relies on the fact that cysteine residues will form disulfide bonds with each other quicker when they are in close proximity. By substituting amino acid residues in PSD-95 for cysteines, Hamilton et al. were able to determine that residues that were presumed to be close together based on the proposed structures formed disulfide bonds faster than randomly selected pairs of residues.
Often, in realtime structural investigations like the ones performed by Hamilton et al., if a protein switches between two conformations faster than the detector can capture, the observed conformation will be a ‘blurred’ average, equally distant from the two extreme protein states (Figure 1D). Previous experiments performed on the PSG supramodule used smFRET to capture slow protein dynamics, and were only able to capture a single structure for the module (McCann et al., 2012). This result was inconsistent with structures for PSD-95 determined using other biophysical methods, such as SAXS or NMR (Zhang et al., 2013).
The observation by Hamilton et al. that PSD-95 fast-switches between its two conformations solves this almost decade-old contradiction: experiments that only capture slow dynamics result in an ‘averaged’ structure of the protein, while capturing the fast dynamics of PSD-95 reveals the two extreme conformations that lead to that average. Hamilton et al. captured both fast and slow protein dynamics using two types of single-molecule FRET microscopes, and their structural model containing two states for PSG now agrees nicely with data from other techniques.
Finally, Hamilton et al. demonstrated that interactions between protein domains in the PSG supramodule are critical for the activity of PSD-95, and, in particular, for its interaction with neuroligin. Negative electric charges from the SH3 domain balance positive charges near the ligand binding pocket of the PDZ3 domain to allow PSD-95 to bind to the positively charged ligand. Additionally, Hamilton et al. showed that structural dynamics of PSG are fine-tuned by the two other domains (PDZ1 and PDZ2). This tuning results in complex local changes of structural dynamics on the timescales from microseconds to seconds.
The techniques used by Hamilton et al. pave the way for future insights into natural interactions between domains in the same protein. This may facilitate the design of more selective drugs targeting inter-domain protein interfaces, as well as de novo design of multi-domain proteins for biotechnological applications. Furthermore, the work of Hamilton et al. builds a solid foundation for further intriguing investigations. For example, how are the conformations and structural dynamics of PSD-95 affected by ligands, protein partners, or post-translational modifications? It will also be important to determine whether there are protein partners and ligands that preferentially bind PSD-95 in the conformation in which the PDZ3 domain directly interacts with the Guk domain? This is, definitely, something for us and the neurons in our brains to learn in the future.
References
-
AMPA receptors and their minions: auxiliary proteins in AMPA receptor traffickingCellular and Molecular Life Sciences 76:2133–2169.https://doi.org/10.1007/s00018-019-03068-7
-
Structural modeling of protein interactions by analogy: application to PSD-95PLOS Computational Biology 2:e153.https://doi.org/10.1371/journal.pcbi.0020153
Article and author information
Author details
Publication history
Copyright
© 2022, Maslov and Hendrix
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,016
- views
-
- 94
- downloads
-
- 2
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Neuroscience
When navigating environments with changing rules, human brain circuits flexibly adapt how and where we retain information to help us achieve our immediate goals.
-
- Neuroscience
When holding visual information temporarily in working memory (WM), the neural representation of the memorandum is distributed across various cortical regions, including visual and frontal cortices. However, the role of stimulus representation in visual and frontal cortices during WM has been controversial. Here, we tested the hypothesis that stimulus representation persists in the frontal cortex to facilitate flexible control demands in WM. During functional MRI, participants flexibly switched between simple WM maintenance of visual stimulus or more complex rule-based categorization of maintained stimulus on a trial-by-trial basis. Our results demonstrated enhanced stimulus representation in the frontal cortex that tracked demands for active WM control and enhanced stimulus representation in the visual cortex that tracked demands for precise WM maintenance. This differential frontal stimulus representation traded off with the newly-generated category representation with varying control demands. Simulation using multi-module recurrent neural networks replicated human neural patterns when stimulus information was preserved for network readout. Altogether, these findings help reconcile the long-standing debate in WM research, and provide empirical and computational evidence that flexible stimulus representation in the frontal cortex during WM serves as a potential neural coding scheme to accommodate the ever-changing environment.






