Articular Cartilage: Where it all started
Articular cartilage is a smooth white tissue that covers the ends of bones where they join together. It is essential for maintaining the mobility of bone joints. However, despite this important role, the tissue is susceptible to degeneration caused by trauma, disease or ageing. This can lead to conditions such as osteoarthritis, which can cause chronic pain and, in some cases, disability (Cui et al., 2020).
Reversing damage to articular cartilage remains a great challenge in musculoskeletal medicine. One way to tackle this issue is to better understand how articular cartilage forms during embryonic development, as this knowledge could help researchers to develop new methods for rebuilding cartilage. Yet, the origins of the main cells in cartilage, known as articular chondrocytes, and the identity of the genes that regulate their production remain unclear. Now, in eLife, Xianpeng Ge (Capital Medical University in Beijing) and colleagues – including Fan Zhang and Yuanyuan Wang as joint first authors – report new insights into the formation of articular chondrocytes (Zhang et al., 2023).
Previous research has shown that the gene NFATc1 helps to regulate the development of bones by controlling cells that absorb and form bone (Winslow et al., 2006). To find out if NFATc1 could also be involved in cartilage development, Zhang et al. used genetically modified mouse embryos, in which cells expressing NFATc1 were tagged with a fluorescent marker that allowed the researchers to track these cells and their offspring over time. This revealed that NFATc1 is expressed in a group of progenitor cells throughout embryonic and even postnatal development, and that these cells are responsible for generating most of the articular chondrocytes (Figure 1).
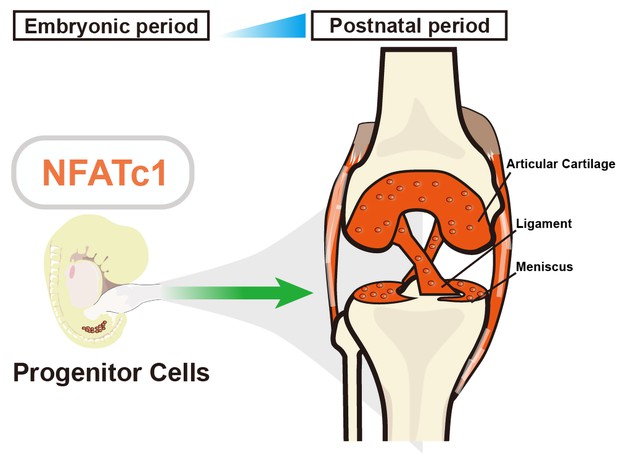
The role of NFATc1 in articular cartilage formation.
Zhang et al. identified a unique progenitor cell population in the knee joint of embryonic mice (left) that expresses a gene called NFATc1, which has previously been linked to bone formation and bone resorption. These progenitor (stem) cells are responsible for generating articular chondrocytes – the main cell type in articular cartilage – during both embryonic and postnatal development (right). Suppressing the activity of NFATc1 in these stem progenitor cells induces the formation of chondrocytes, which are essential for forming articular cartilage.
Further experiments in cells and embryonic mice revealed that the expression of NFATc1 decreased as the articular chondrocytes matured. Zhang et al. found that when the gene was removed from the cartilage progenitors, the maturation of chondrocytes was faster and the formation of articular cartilage in embryos was still supported. Consistent with this, when NFATc1 was overactivated, the progenitor cells could no longer mature into chondrocytes. This indicates that this gene negatively regulates the development of articular chondrocytes.
So far, it has been challenging to track how articular cartilage develops as most genetic markers cannot distinguish between the different cell types in the relevant tissues (Rux et al., 2019; Chijimatsu and Saito, 2019). The only exception is the gene Prg4, a widely used marker of articular cartilage progenitors. But since cells only start to express this gene at a later stage during development, the marker fails to track their movement during the earlier stages (Kozhemyakina et al., 2015). In contrast, NFATc1 is expressed early during articular cartilage development, and may therefore offer a better alternative for studying the origin of articular cartilage.
It was previously thought that NFATc1, together with another gene involved in cartilage formation, NFATc2, restricts the overgrowth of cartilage (also known as osteochondroma) at the sites where the ligaments insert into the bone. However, adjusting how much of either of those genes was removed from the mice’s progenitor cells could in fact determine the number and size of osteochondromas (Ge et al., 2016). This knowledge may be helpful in understanding how NFATc1 may suppress the formation of cartilage in certain diseases.
It has been suggested that both NFATc1 and NFATc2 are key suppressors of osteoarthritis, which is crucial for articular cartilage formation in mice (Greenblatt et al., 2013). However, when Zhang et al. used a different approach to delete these two genes, they observed the opposite effect – removing NFATc1 and NFATc2 enhanced chondrogenesis under pathological conditions. This suggests that researchers should carefully consider the genetic ablating tools they use when studying the role of progenitor cells in articular cartilage.
Moreover, it has been shown that adult skeletal stem cells expressing NFATc1 behave as bone-cell progenitors and contribute to the early stages of repair following a bone fracture (Yu et al., 2022). This highlights the complex pool of progenitor cells that express NFATc1, and how the fate of these progenitors changes during different periods of development as well as adulthood. More importantly, to date, it remains unclear whether these NFATc1-expressing progenitor cells fulfill stemness criteria in the skeleton, such as stem-cell transplantation (Debnath et al., 2018), or if these cells are able to generate functional articular cartilage.
Despite a lack of evidence confirming the stemness of NFATc1-expressing progenitor cells, the work of Zhang et al. offers researchers an additional genetic tool to detect and track the origins of articular chondrocytes. Moreover, their findings provide valuable insights into the mechanism of articular cartilage formation. If studies in human cells achieve similar results, this may help scientists to identify potential treatments of cartilage diseases, such as osteoarthritis.
References
-
Mechanisms of synovial joint and articular cartilage developmentCellular and Molecular Life Sciences 76:3939–3952.https://doi.org/10.1007/s00018-019-03191-5
-
Identification of a Prg4-expressing articular cartilage progenitor cell population in miceArthritis & Rheumatology 67:1261–1273.https://doi.org/10.1002/art.39030
-
Joints in the appendicular skeleton: developmental mechanisms and evolutionary influencesCurrent Topics in Developmental Biology 133:119–151.https://doi.org/10.1016/bs.ctdb.2018.11.002
-
Calcineurin/NFAT signaling in osteoblasts regulates bone massDevelopmental Cell 10:771–782.https://doi.org/10.1016/j.devcel.2006.04.006
Article and author information
Author details
Publication history
Copyright
© 2023, Zhang et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 630
- views
-
- 65
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Medicine
- Neuroscience
Neurodegenerative diseases are age-related disorders characterized by the cerebral accumulation of amyloidogenic proteins, and cellular senescence underlies their pathogenesis. Thus, it is necessary for preventing these diseases to remove toxic proteins, repair damaged neurons, and suppress cellular senescence. As a source for such prophylactic agents, we selected zizyphi spinosi semen (ZSS), a medicinal herb used in traditional Chinese medicine. Oral administration of ZSS hot water extract ameliorated Aβ and tau pathology and cognitive impairment in mouse models of Alzheimer’s disease and frontotemporal dementia. Non-extracted ZSS simple crush powder showed stronger effects than the extract and improved α-synuclein pathology and cognitive/motor function in Parkinson’s disease model mice. Furthermore, when administered to normal aged mice, the ZSS powder suppressed cellular senescence, reduced DNA oxidation, promoted brain-derived neurotrophic factor expression and neurogenesis, and enhanced cognition to levels similar to those in young mice. The quantity of known active ingredients of ZSS, jujuboside A, jujuboside B, and spinosin was not proportional to the nootropic activity of ZSS. These results suggest that ZSS simple crush powder is a promising dietary material for the prevention of neurodegenerative diseases and brain aging.
-
- Medicine
- Neuroscience
Monomethyl fumarate (MMF) and its prodrug dimethyl fumarate (DMF) are currently the most widely used agents for the treatment of multiple sclerosis (MS). However, not all patients benefit from DMF. We hypothesized that the variable response of patients may be due to their diet. In support of this hypothesis, mice subjected to experimental autoimmune encephalomyelitis (EAE), a model of MS, did not benefit from DMF treatment when fed a lauric acid-rich (LA) diet. Mice on normal chow (NC) diet, in contrast, and even more so mice on high-fiber (HFb) diet showed the expected protective DMF effect. DMF lacked efficacy in the LA diet-fed group despite similar resorption and preserved effects on plasma lipids. When mice were fed the permissive HFb diet, the protective effect of DMF treatment depended on hydroxycarboxylic receptor 2 (HCAR2) which is highly expressed in neutrophil granulocytes. Indeed, deletion of Hcar2 in neutrophils abrogated DMF protective effects in EAE. Diet had a profound effect on the transcriptional profile of neutrophils and modulated their response to MMF. In summary, DMF required HCAR2 on neutrophils as well as permissive dietary effects for its therapeutic action. Translating the dietary intervention into the clinic may improve MS therapy.






