Gene Expression: How their environment influences endothelial cells
Cell culture is a widely used technique in biology (Jensen and Teng, 2020), and cells from the endothelium – the single layer of cells that lines the inside of blood vessels – are often employed in cardiovascular research. Endothelial cells are unique in that they are in direct contact with the blood, and the flow of blood exerts a mechanical force (known as shear stress) that can activate signaling pathways and modify gene expression in the cells. Moreover, endothelial cells are also close to smooth muscle cells (Figure 1), and these two types of cells communicate with each other to maintain healthy blood vessels (Li et al., 2018).
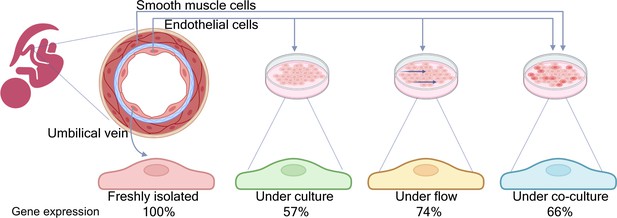
Comparing gene expression profiles in endothelial cells in vivo and under different in vitro conditions.
Schematic cross-section of an umbilical vein (top left) made up of a single inner layer of endothelial cells surrounded by a basement membrane (blue) and multiple layers of smooth muscle cells. Afshar et al. compared the gene expression profiles of four samples of endothelial cells: cells freshly isolated from human umbilical cords (left); cord cells cultured in standard conditions; cord cells cultured under flow; and cord cells co-cultured with smooth muscle cells from the same donor (right). Under standard culture conditions, around 57% of the genes expressed in vivo showed no significant changes in vitro. This percentage increased to 74% when the cells were cultured under flow conditions, and to 66% when the endothelial cells were co-cultured with smooth muscle cells.
Image credit: Figure created with BioRender.
Most of the endothelial cells used in cell culture experiments are taken from the umbilical cord of newborn babies, but we do not fully understand how removing these cells from their native environment impacts their behavior and function (Aird, 2007). In particular, it is not clear how the absence of two factors – shear stress and communication between different cell types – changes the behavior of endothelial cells in cell culture experiments. Furthermore, is it possible to change the conditions in cell culture experiments to make them more similar to the conditions in vivo?
Now, in eLife, Luisa Iruela-Arispe (Northwestern University) and colleagues – including Yalda Afshar (University of California) as first author – report that gene expression in endothelial cells is altered when the cells are cultured in vitro, and can be partially restored if the cells are exposed to shear stress and smooth muscle cells (Afshar et al., 2023).
Using endothelial cells taken from the umbilical cords of seven human donors, Afshar et al. compared the gene expression profiles of cells cultured under in vitro conditions with the profiles of cells freshly isolated from the same donor (hereafter called cord cells). RNA sequencing showed that nearly half of the genes expressed in the culture condition were different from those expressed in the freshly isolated cells: in particular, genes sensitive to blood flow were expressed less, and genes related to cell proliferation were expressed more (Figure 1).
Afshar et al. then modified the culture conditions to see if the gene expression profile could be made more similar to the profile of the cord cells. First, the cultured cells were placed under flow conditions for 48 hours to simulate the shear stress caused by blood flow (Chiu and Chien, 2011). This led to the expression of a number of genes that were not expressed in culture without flow, including genes which belong to two signaling pathways – BMP and NOTCH – that are known to be sensitive to flow (Souilhol et al., 2020).
Next, Afshar et al. co-cultured endothelial cells alongside smooth muscle cells from the same donor. Single-cell RNA sequencing showed that this restored the expression of various genes (including genes for cytoskeleton proteins) that were not expressed when endothelial cells were cultured on their own. Moreover, the expression of genes related to cell proliferation was reduced. Furthermore, the team also built “Flow Profiler”, an open-source website to display the analyzed datasets and allow further exploration of the behavior of genes under flow conditions.
The results of Afshar et al. demonstrate the impact of culture conditions on gene expression in a systematic and quantitative fashion, and highlight the importance of contextual information when interpreting experimental results. Going forward it would be interesting to explore if introducing flow and smooth muscle cells at the same time would restore even more of the gene expression profile. Furthermore, endothelial cells are found in a variety of vessels in the body, and the flow pattern (and hence the shear stress) will be different in different vessels: future work could explore the impact of different locations and flow patterns on gene expression profiles. It would also be interesting to investigate the influence of DNA methylation and histone modification on gene expression in endothelial cells. The knowledge and insights gained from such studies will help researchers to develop more representative cell culture models for the study of cells and organs.
Researchers are also exploring a range of other techniques that mimic the in vivo environment, such as 3D bioprinting (Neufeld et al., 2022), organoids (LeSavage et al., 2022) and organ-on-a-chip technologies (Tolabi et al., 2023). Combining all these techniques, including improved cell culture models, should lead to a better understanding of cell behavior and disease mechanisms, and thus help researchers working to improve medical outcomes in scenarios where endothelial function is impaired.
References
-
Is it time to start transitioning from 2D to 3D cell culture?Frontiers in Molecular Biosciences 7:33.https://doi.org/10.3389/fmolb.2020.00033
-
Next-generation cancer organoidsNature Materials 21:143–159.https://doi.org/10.1038/s41563-021-01057-5
-
Endothelial-vascular smooth muscle cells interactions in atherosclerosisFrontiers in Cardiovascular Medicine 5:151.https://doi.org/10.3389/fcvm.2018.00151
-
3D bioprinted cancer models: from basic biology to drug developmentNature Reviews Cancer 22:679–692.https://doi.org/10.1038/s41568-022-00514-w
-
Endothelial responses to shear stress in atherosclerosis: a novel role for developmental genesNature Reviews Cardiology 17:52–63.https://doi.org/10.1038/s41569-019-0239-5
Article and author information
Author details
Publication history
Copyright
© 2023, Liu and Bouman Chen
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,618
- views
-
- 143
- downloads
-
- 4
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Cancer Biology
- Cell Biology
Testicular microcalcifications consist of hydroxyapatite and have been associated with an increased risk of testicular germ cell tumors (TGCTs) but are also found in benign cases such as loss-of-function variants in the phosphate transporter SLC34A2. Here, we show that fibroblast growth factor 23 (FGF23), a regulator of phosphate homeostasis, is expressed in testicular germ cell neoplasia in situ (GCNIS), embryonal carcinoma (EC), and human embryonic stem cells. FGF23 is not glycosylated in TGCTs and therefore cleaved into a C-terminal fragment which competitively antagonizes full-length FGF23. Here, Fgf23 knockout mice presented with marked calcifications in the epididymis, spermatogenic arrest, and focally germ cells expressing the osteoblast marker Osteocalcin (gene name: Bglap, protein name). Moreover, the frequent testicular microcalcifications in mice with no functional androgen receptor and lack of circulating gonadotropins are associated with lower Slc34a2 and higher Bglap/Slc34a1 (protein name: NPT2a) expression compared with wild-type mice. In accordance, human testicular specimens with microcalcifications also have lower SLC34A2 and a subpopulation of germ cells express phosphate transporter NPT2a, Osteocalcin, and RUNX2 highlighting aberrant local phosphate handling and expression of bone-specific proteins. Mineral disturbance in vitro using calcium or phosphate treatment induced deposition of calcium phosphate in a spermatogonial cell line and this effect was fully rescued by the mineralization inhibitor pyrophosphate. In conclusion, testicular microcalcifications arise secondary to local alterations in mineral homeostasis, which in combination with impaired Sertoli cell function and reduced levels of mineralization inhibitors due to high alkaline phosphatase activity in GCNIS and TGCTs facilitate osteogenic-like differentiation of testicular cells and deposition of hydroxyapatite.
-
- Cell Biology
- Genetics and Genomics
A glaucoma polygenic risk score (PRS) can effectively identify disease risk, but some individuals with high PRS do not develop glaucoma. Factors contributing to this resilience remain unclear. Using 4,658 glaucoma cases and 113,040 controls in a cross-sectional study of the UK Biobank, we investigated whether plasma metabolites enhanced glaucoma prediction and if a metabolomic signature of resilience in high-genetic-risk individuals existed. Logistic regression models incorporating 168 NMR-based metabolites into PRS-based glaucoma assessments were developed, with multiple comparison corrections applied. While metabolites weakly predicted glaucoma (Area Under the Curve = 0.579), they offered marginal prediction improvement in PRS-only-based models (p=0.004). We identified a metabolomic signature associated with resilience in the top glaucoma PRS decile, with elevated glycolysis-related metabolites—lactate (p=8.8E-12), pyruvate (p=1.9E-10), and citrate (p=0.02)—linked to reduced glaucoma prevalence. These metabolites combined significantly modified the PRS-glaucoma relationship (Pinteraction = 0.011). Higher total resilience metabolite levels within the highest PRS quartile corresponded to lower glaucoma prevalence (Odds Ratiohighest vs. lowest total resilience metabolite quartile=0.71, 95% Confidence Interval = 0.64–0.80). As pyruvate is a foundational metabolite linking glycolysis to tricarboxylic acid cycle metabolism and ATP generation, we pursued experimental validation for this putative resilience biomarker in a human-relevant Mus musculus glaucoma model. Dietary pyruvate mitigated elevated intraocular pressure (p=0.002) and optic nerve damage (p<0.0003) in Lmx1bV265D mice. These findings highlight the protective role of pyruvate-related metabolism against glaucoma and suggest potential avenues for therapeutic intervention.






