Arthritis: Unraveling the mechanisms behind joint damage
Rheumatoid arthritis is an inflammatory autoimmune disease in which the immune system gradually destroys the lining of the joints, leading to pain, swelling and stiffness in the affected regions (Komatsu and Takayanagi, 2022; Weyand and Goronzy, 2021). A key part of disease progression is the transition from autoimmunity to the destruction of the joint. In this process, osteoclasts – cells that break down bone to maintain joint homeostasis – become overactive, leading to the destruction of healthy bone tissue (Fu et al., 2021; Meehan et al., 2021). A complex network of signaling mechanisms drives the formation of osteoclasts from the fusion of immune cells called monocytes. Autoimmunity increases the expression of key molecules in this process, which is thought to drive formation of mature osteoclasts and further joint destruction.
There is currently no cure for this condition, and it remains unclear what exactly triggers the immune system to attack the body, and how this leads to the progressive destruction of the joints. Understanding the mechanisms leading to joint deterioration is thus crucial for the development of effective treatments for rheumatoid arthritis (Fearon et al., 2022; Tang et al., 2011).
Now, in eLife, Xu Cao and colleagues at the Johns Hopkins University School of Medicine – including Weixin Zhang and Kathleen Noller as joint first authors – report that a specific subpopulation of monocytes drives the transition from autoimmunity to joint destruction in rheumatoid arthritis (Zhang et al., 2023).
Using a widely accepted mouse model of rheumatoid arthritis, Zhang et al. carried out a technique called single-cell RNA sequencing to take a closer look at the signaling pathways underlying this change. The experiments revealed that monocytes in these mice overexpress two receptor proteins, TLR2 and RANK, which are important for recognizing foreign substances and initiating an immune response, and for the development of osteoclasts.
Moreover, fluorescent imaging showed increased sialylation, the addition of sialic acids, at specific sites on TLR2 in these RANK+TLR2+ cells. This was driven by increased expression of enzymes responsible for sialylation in monocytes.
When either the enzymes or TLR2 were experimentally blocked, the monocytes did not transform to become osteoclasts. Consequently, the bone was not resorbed, and the destruction of the joints was mitigated (Figure 1). These findings further support the results of recent research suggesting that the sialylation of TLR2 has a role in initiating cell fusion during osteoclast formation (Dou et al., 2022).
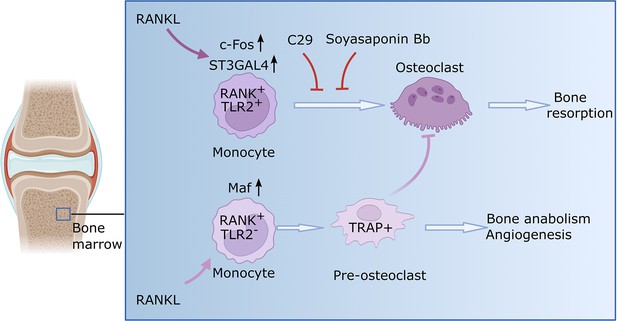
Osteoclast formation in the context of rheumatoid arthritis.
Monocytes are a type of immune cell (light purple shape) that can develop into osteoclasts (dark purple shape), cells that can degrade healthy bone to initiate remodeling. In certain conditions, such as rheumatoid arthritis, the activity of osteoclasts is increased, which leads to progressive bone loss. Zhang et al. identified two subsets of monocytes. One subset (RANK+TLR2+) overexpressed two relevant signaling molecules, RANK and TLR2, and is involved in bone resorption. When RANK is activated by RANKL in this subset, the enzyme responsible for TLR2 sialylation (ST3GAL4) becomes upregulated through a molecule called c-Fos (a transcription factor critical for the regulation of osteoclast differentiation). Consequently, TLR2 undergoes sialylation, a process that adds sialic acids that enable TLR2 to bind with different molecules in order to allow fusion of cells. Blocking sialyation or TLR2 with specific molecules (C29 and soyasaponin Bb, respectively) prevents mature osteoclasts from forming and the bone remains intact. The other monocyte subset (RANK+TLR2-) exhibited high levels of RANK but low levels of TLR2. In this subset, RANKL prompts the monocytes to differentiate into pre-osteoclasts that express a molecule called TRAP+, but they fail to mature into fully-functional osteoclasts. Instead, these cells have anabolic properties and promote the production of new bone and blood vessels. This figure was generated using BioRender.com.
Single-cell RNA sequencing further revealed a previously unknown subset of myeloid monocytes, which overexpress RANK but do not express TLR2. These RANK+TLR2- monocytes were able to differentiate into precursor osteoclasts, but they were unable to fuse and mature into fully functional osteoclasts (Figure 1). They promoted, however, the expression of proteins that further the formation of new blood vessels and bones, which may explain presence of precursor osteoclasts displaying anabolic properties in bone (i.e., with the ability to promote the biosynthesis that further tissue growth).
Zhang et al. provide crucial insights into the mechanisms driving the transition from the onset of autoimmunity to joint destruction in rheumatoid arthritis. The findings emphasize the central role of myeloid monocytes that overexpress both RANK and TLR2, and the sialylation of TLR2 in driving the fusion of monocytes and consequent resorption of bone. Targeting the sialylation process in these monocytes represents a promising avenue for preventing autoimmune-mediated joint destruction and preserving bone health in patients with rheumatoid arthritis, and potentially other autoimmune disorders. Moreover, the identification of monocytes that lack TLR2 offers valuable insights into the complex cellular dynamics involved in joint destruction and may open new avenues for exploring the potential of these cells in promoting bone repair and regeneration. More research is needed to better understand if and how the two monocyte subsets interact and their clinical relevance in humans.
In summary, the potential therapeutic targets identified in this study should help with efforts to develop drugs that prevent autoimmune-mediated joint destruction and promote bone production in rheumatoid arthritis and various autoimmune diseases.
References
-
Cellular metabolic adaptations in rheumatoid arthritis and their therapeutic implicationsNature Reviews Rheumatology 18:398–414.https://doi.org/10.1038/s41584-022-00771-x
-
TNFR2/14-3-3Ε signaling complex instructs macrophage plasticity in inflammation and autoimmunityThe Journal of Clinical Investigation 131:e144016.https://doi.org/10.1172/JCI144016
-
Mechanisms of joint destruction in rheumatoid arthritis - immune cell-fibroblast-bone interactionsNature Reviews Rheumatology 18:415–429.https://doi.org/10.1038/s41584-022-00793-5
-
Preclinical models of arthritis for studying immunotherapy and immune toleranceAnnals of the Rheumatic Diseases 80:1268–1277.https://doi.org/10.1136/annrheumdis-2021-220043
-
The immunology of rheumatoid arthritisNature Immunology 22:10–18.https://doi.org/10.1038/s41590-020-00816-x
Article and author information
Author details
Publication history
Copyright
© 2023, Fu and Liu
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 473
- views
-
- 42
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Medicine
Background:
Approximately one-third of patients with HER2-positive breast cancer experienced recurrence within 10 years after receiving 1 year of adjuvant trastuzumab. The ExteNET study showed that 1 year of extended adjuvant neratinib after trastuzumab-based adjuvant therapy could reduce invasive disease-free survival (iDFS) events compared with placebo. This study investigated the efficacy and safety of pyrotinib, an irreversible pan-HER receptor tyrosine kinase inhibitor, after trastuzumab-based adjuvant therapy in patients with high-risk, HER2-positive early or locally advanced breast cancer.
Methods:
This multicenter phase II trial was conducted at 23 centers in China. After enrollment, patients received 1 year of extended adjuvant pyrotinib (400 mg/day), which should be initiated within 6 months after the completion of 1-year adjuvant therapy (trastuzumab alone or plus pertuzumab). The primary endpoint was 2-year iDFS rate.
Results:
Between January 2019 and February 2022, 141 eligible women were enrolled and treated. As of October 10, 2022, the median follow-up was 24 (interquartile range, 18.0–34.0) months. The 2-year iDFS rate was 94.59% (95% confidence interval [CI]: 88.97–97.38) in all patients, 94.90% (95% CI: 86.97–98.06) in patients who completed 1-year treatment, 90.32% (95% CI: 72.93–96.77) in patients who completed only 6-month treatment, 96.74% (95% CI: 87.57–99.18) in the hormone receptor (HR)-positive subgroup, 92.77% (95% CI: 83.48–96.93) in the HR-negative subgroup, 96.88% (95% CI: 79.82–99.55) in the lymph node-negative subgroup, 93.85% (95% CI: 86.81–97.20) in the lymph node-positive subgroup, 97.30% (95% CI: 82.32–99.61) in patients with adjuvant trastuzumab plus pertuzumab, and 93.48% (95% CI: 86.06–97.02) in patients with adjuvant trastuzumab. The most common adverse events were diarrhea (79.4%), fatigue (36.9%), lymphocyte count decreased (36.9%), nausea (33.3%), and hand-foot syndrome (33.3%).
Conclusions:
Extended adjuvant pyrotinib administrated after trastuzumab-based adjuvant therapy showed promising efficacy in patients with high-risk HER2-positive breast cancer. The follow-up is ongoing to determine the long-term benefit.
Funding:
No external funding was received for this work.
Clinical trial number:
ClinicalTrials.gov: NCT05880927
-
- Immunology and Inflammation
- Medicine
Preeclampsia (PE), a major cause of maternal and perinatal mortality with highly heterogeneous causes and symptoms, is usually complicated by gestational diabetes mellitus (GDM). However, a comprehensive understanding of the immune microenvironment in the placenta of PE and the differences between PE and GDM is still lacking. In this study, cytometry by time of flight indicated that the frequencies of memory-like Th17 cells (CD45RA−CCR7+IL-17A+CD4+), memory-like CD8+ T cells (CD38+CXCR3−CCR7+Helios−CD127−CD8+) and pro-inflam Macs (CD206−CD163−CD38midCD107alowCD86midHLA-DRmidCD14+) were increased, while the frequencies of anti-inflam Macs (CD206+CD163−CD86midCD33+HLA-DR+CD14+) and granulocyte myeloid-derived suppressor cells (gMDSCs, CD11b+CD15hiHLA-DRlow) were decreased in the placenta of PE compared with that of normal pregnancy (NP), but not in that of GDM or GDM&PE. The pro-inflam Macs were positively correlated with memory-like Th17 cells and memory-like CD8+ T cells but negatively correlated with gMDSCs. Single-cell RNA sequencing revealed that transferring the F4/80+CD206− pro-inflam Macs with a Folr2+Ccl7+Ccl8+C1qa+C1qb+C1qc+ phenotype from the uterus of PE mice to normal pregnant mice induced the production of memory-like IL-17a+Rora+Il1r1+TNF+Cxcr6+S100a4+CD44+ Th17 cells via IGF1–IGF1R, which contributed to the development and recurrence of PE. Pro-inflam Macs also induced the production of memory-like CD8+ T cells but inhibited the production of Ly6g+S100a8+S100a9+Retnlg+Wfdc21+ gMDSCs at the maternal–fetal interface, leading to PE-like symptoms in mice. In conclusion, this study revealed the PE-specific immune cell network, which was regulated by pro-inflam Macs, providing new ideas about the pathogenesis of PE.






