Tuberculosis Vaccine: Dual delivery of antigens shows promise
The BCG vaccine was first used in 1921 and, remarkably, over 100 years later it is the only licensed vaccine for use against tuberculosis. While the BCG vaccine provides protection against tuberculosis that has spread beyond the lungs in young children, its efficacy against lung infections in adults – the main form of the disease – is variable (Trunz et al., 2006). Tuberculosis is caused by a bacterium called Mycobacterium tuberculosis (M. tb), and one reason for the lack of more effective vaccines is that we still do not know which M. tb antigens are able to induce protective immunity (Guinn and Rubin, 2017).
Almost 20 vaccine candidates are currently being evaluated in clinical trials. Most of these aim to induce a response from immune cells called type 1 helper T cells by exposing them to protein antigens from M. tb, although other approaches are also being explored. Now, in eLife, Evan Scott and Chyung-Ru Wang from Northwestern University and colleagues – including Eva Morgun as first author – report a new approach that involves using protein antigens and long-chain fatty acids called mycolic acids, found in the M. tb cell envelope, to induce an immune response (Morgun et al., 2023).
Previous studies have shown that the immune system generates a protective response when exposed to mycolic acids: this response is mediated by a subset of “unconventional” T cells, but the details of the response are not fully understood (Shang et al., 2018; Zhao et al., 2015). Moreover, there have been very few attempts to explore the use of mycolic acids as alternative antigens for a tuberculosis vaccine, partly because most vaccine delivery systems are designed for hydrophilic cargoes (such as proteins), whereas mycolic acids are hydrophobic. Morgun et al. have overcome this problem by developing a system that can deliver hydrophobic and hydrophilic cargoes at the same time.
Building upon evidence that vaccine formulations including M. tb lipids encapsulated in liposomes – hollow spherical structures made of lipid bilayers – provide protection (Dascher et al., 2003; Larrouy-Maumus et al., 2017), Morgun et al. developed structures called bicontinuous nanospheres. These self-assembled polymer-based structures can carry both hydrophobic and hydrophilic components because they possess an organized internal cubic structure including hydrophobic bilayers and hydrophilic aqueous channels (Figure 1). Furthermore, the nanospheres retain antigens more efficiently than other lipid nanostructures, and only release their contents when they reach the target compartment within antigen-presenting cells. These features increase the persistence of the antigens within cells and stimulate the immune system for longer periods of time, which leads to a more robust immune response (Correia-Pinto et al., 2013).
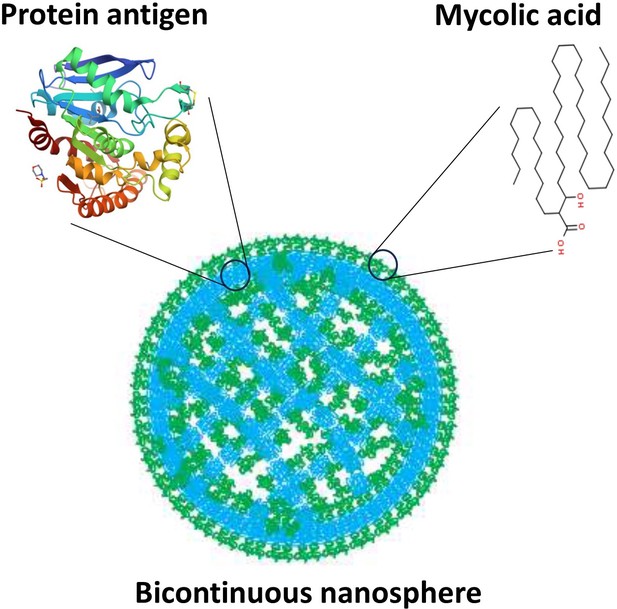
Bicontinuous nanospheres can be loaded with both protein and lipid antigens.
Structure of a bicontinuous nanosphere. Hydrophilic (blue) regions contain protein antigens and hydrophobic (green) regions contain mycolic acids, represented by tentative structures. Figure based on image from Allen et al., 2018.
Morgun et al. used a mouse model that allowed them to observe the immune response to dual vaccination with mycolic acids and proteins. This showed that T cells responded to both antigens: however, only mycolic acid remained detectable inside immune cells after vaccination. It remains unclear if this differential antigen persistence is due to the nanosphere delivery system, the protein itself, or a distinct mechanism of antigen capture and maintenance by immune cells.
It is worth noting that the persistence of mycolic acids likely occurs during in vivo infection and, therefore, the dual vaccination strategy may mimic a previously overlooked feature of the infection process. Supporting this notion, Morgun et al. found that vaccination with an attenuated M. tb strain resulted in a persistence of mycolic acids similar to that observed with the dual approach. These results suggest that vaccines using live attenuated strains of M. tb likely provide protection via mycolic acid-specific T cells. This raises the question of whether the persistence of lipid antigens is a key aspect of the protective immune response: this is something that needs to be considered when developing tuberculosis vaccines in the future.
Although definitive proof of protective immunity induced by dual-loaded bicontinuous nanospheres remains elusive, this approach allowing combinations of antigens – both hydrophobic and hydrophilic – can be used as an alternative strategy to live attenuated vaccines. Moreover, the ability to test combinations of alternative antigens will aid the development of vaccines for tuberculosis more generally. Future work with this system could also investigate the role of lipid persistence in other overlooked aspects of tuberculosis immunity, such as antibody responses, which might have an important contribution to protective immunity.
References
-
Vaccine delivery carriers: insights and future perspectivesInternational Journal of Pharmaceutics 440:27–38.https://doi.org/10.1016/j.ijpharm.2012.04.047
Article and author information
Author details
Publication history
Copyright
© 2023, Luque-García and Prados-Rosales
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 647
- views
-
- 57
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Immunology and Inflammation
The incidence of metabolic dysfunction-associated steatotic liver disease (MASLD) has been increasing worldwide. Since gut-derived bacterial lipopolysaccharides (LPS) can travel via the portal vein to the liver and play an important role in producing hepatic pathology, it seemed possible that (1) LPS stimulates hepatic cells to accumulate lipid, and (2) inactivating LPS can be preventive. Acyloxyacyl hydrolase (AOAH), the eukaryotic lipase that inactivates LPS and oxidized phospholipids, is produced in the intestine, liver, and other organs. We fed mice either normal chow or a high-fat diet for 28 weeks and found that Aoah-/- mice accumulated more hepatic lipid than did Aoah+/+ mice. In young mice, before increased hepatic fat accumulation was observed, Aoah-/- mouse livers increased their abundance of sterol regulatory element-binding protein 1, and the expression of its target genes that promote fatty acid synthesis. Aoah-/- mice also increased hepatic expression of Cd36 and Fabp3, which mediate fatty acid uptake, and decreased expression of fatty acid-oxidation-related genes Acot2 and Ppara. Our results provide evidence that increasing AOAH abundance in the gut, bloodstream, and/or liver may be an effective strategy for preventing or treating MASLD.
-
- Immunology and Inflammation
- Microbiology and Infectious Disease
Pseudomonas aeruginosa (PA) is an opportunistic, frequently multidrug-resistant pathogen that can cause severe infections in hospitalized patients. Antibodies against the PA virulence factor, PcrV, protect from death and disease in a variety of animal models. However, clinical trials of PcrV-binding antibody-based products have thus far failed to demonstrate benefit. Prior candidates were derivations of antibodies identified using protein-immunized animal systems and required extensive engineering to optimize binding and/or reduce immunogenicity. Of note, PA infections are common in people with cystic fibrosis (pwCF), who are generally believed to mount normal adaptive immune responses. Here, we utilized a tetramer reagent to detect and isolate PcrV-specific B cells in pwCF and, via single-cell sorting and paired-chain sequencing, identified the B cell receptor (BCR) variable region sequences that confer PcrV-specificity. We derived multiple high affinity anti-PcrV monoclonal antibodies (mAbs) from PcrV-specific B cells across three donors, including mAbs that exhibit potent anti-PA activity in a murine pneumonia model. This robust strategy for mAb discovery expands what is known about PA-specific B cells in pwCF and yields novel mAbs with potential for future clinical use.






