Endogenous oligomer formation underlies DVL2 condensates and promotes Wnt/β-catenin signaling
Figures
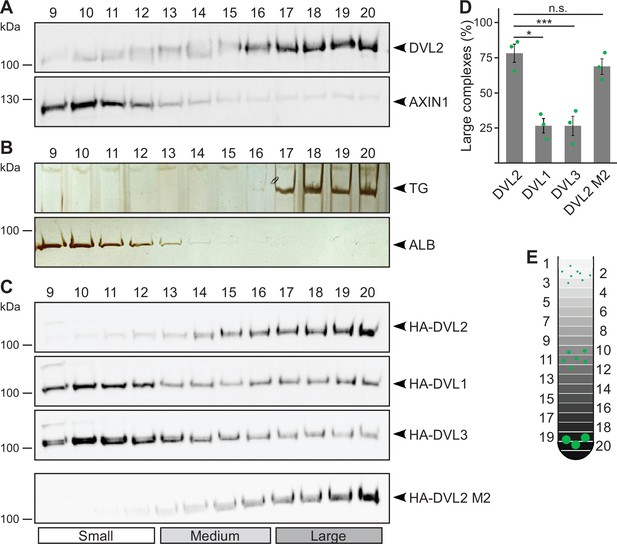
Endogenous dishevelled 2 (DVL2) forms paralog-specific oligomeric complexes.
(A,C) Western blotting for indicated endogenous (A) or transiently expressed proteins, detected with anit-HA antibodies (C) after fractionation of HEK293T cell lysates via sucrose density ultracentrifugation. (B) Silver staining of thyroglobulin (TG) or albumin (ALB) after sucrose density ultracentrifugation of purified proteins. (A-C) shows one out of at least three representative experiments. Analyzed fractions are indicated above the blots according to (E). (D) Amount of the protein that was engaged in large complexes (see label Large in C), relative to the cumulative protein amount detected in all investigated fractions, as determined by 2D densitometry analysis of protein bands from three independent experiments as in (C) (n=3). Results are mean ± SEM, *p<0.05, ***p<0.001 (Student’s t-test). (E) Schematic representation of the ultracentrifugation assay, illustrating the distribution of proteins of different sizes (green) within numbered fractions of a sucrose gradient form low (light gray) to high density (black).
-
Figure 1—source data 1
Excel file providing the numerical source data to Figure 1.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig1-data1-v1.xlsx
-
Figure 1—source data 2
PDF files containing the original, labeled blots and gels to Figure 1.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig1-data2-v1.zip
-
Figure 1—source data 3
TIF files of the raw blots and gels to Figure 1.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig1-data3-v1.zip

Endogenous dishevelled 2 (DVL2) forms complexes.
(A, B) Western blotting for indicated endogenous proteins after fractionation of U2OS (A) or HEK293T cell lysates (B) via sucrose density ultracentrifugation. Fraction numbers are indicated above the blots according to Figure 1E. (C) Western blotting for indicated endogenous proteins in lysates of HEK293T cells, which were transfected with a control or a DVL2-targeting siRNA for 48 hr. (A–C) Two different antibodies were used for DVL2 detection (3224 and 3216, Cell Signaling). (D) Clustal Omega alignment of indicated dishevelled protein sequences, with specific UniProt identifiers provided in brackets. Identity (*) and conservation between amino acid groups of strongly (:) and weakly (.) similar properties are indicated (Sievers et al., 2011). Marks show conserved domains (CD1 and CD2), low-complexity regions (LCR1, LCR2, LCR3, and LCR4) according to the SEG algorithm (Wootton and Federhen, 1993) and the aggregation propensity of individual residues of mouse DVL2 (yellow-orange heat map) according to the TANGO algorithm (Fernandez-Escamilla et al., 2004). LCR4 and CD2 are highlighted in green and magenta, respectively, as they were identified as crucial regions for condensate induction later on.
-
Figure 1—figure supplement 1—source data 1
PDF files containing the original, labeled blots to Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig1-figsupp1-data1-v1.zip
-
Figure 1—figure supplement 1—source data 2
TIF files of the raw blots to Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig1-figsupp1-data2-v1.zip
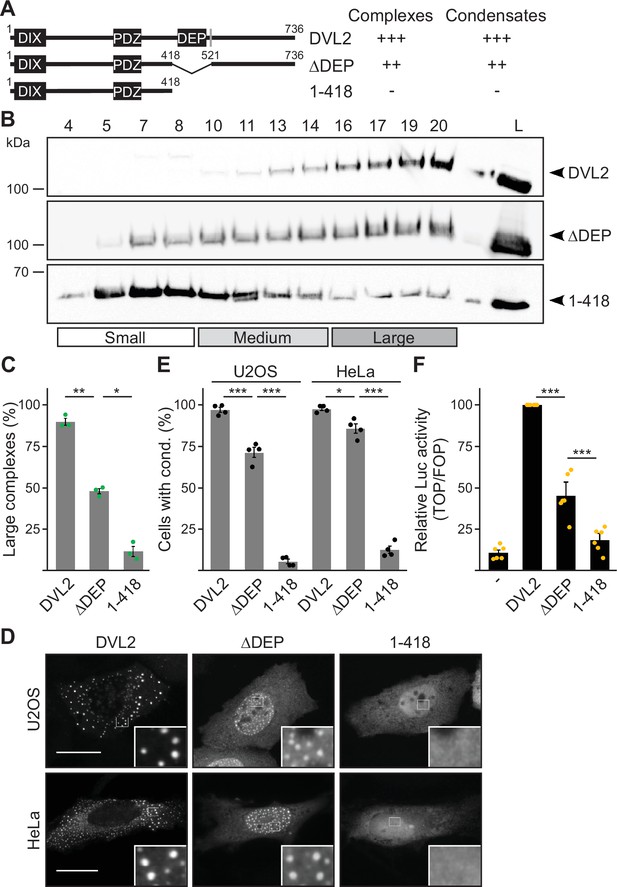
The dishevelled 2 (DVL2) C-terminus promotes complexes, condensates, and activity.
(A) To scale schemes of DVL2 constructs with the DIX, the PDZ, and the DEP domain. A nuclear export signal is highlighted in gray (Itoh et al., 2005). Indicated complexation and condensation summarizes the findings in (B–E). (B) Western blotting for indicated transiently expressed proteins bevor (L) and after fractionation (4-20) of HEK293T cell lysates via sucrose density ultracentrifugation. (C) Percentage of the protein that was engaged in large complexes as specified in (B) (n=3, refer to the legend in Figure 1D for more details). (D) Immunofluorescence of indicated HA-tagged proteins in transiently transfected U2OS and HeLa cells. Scale bars: 20 µm. Insets are magnifications of the boxed areas. Interestingly, DEP domain deleted constructs frequently showed nuclear condensates in contrast to the cytosolic condensates of full length DVL2, which is most likely explained by the deletion of a nearby nuclear export signal (see A) and which still allowed determining differences in condensation capacity. (E) Percentage of cells with condensates out of 1200 transfected cells from four independent experiments as in (D) (n=4). (F) Relative luciferase activity reporting β-catenin-dependent transcription in HEK293T cells expressing the indicated constructs (n=6). (C, E, F) Results are mean ± SEM, *p<0.05, **p<0.01, ***p<0.001 (Student’s t-test).
-
Figure 2—source data 1
Excel file providing the numerical source data to Figure 2.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig2-data1-v1.xlsx
-
Figure 2—source data 2
PDF file containing the original, labeled blots to Figure 2.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig2-data2-v1.zip
-
Figure 2—source data 3
TIF files of the raw blots to Figure 2.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig2-data3-v1.zip
A 58 aa C-terminal region promotes dishevelled 2 (DVL2) condensates and activity.
(A) To scale schemes of DVL2 constructs. Indicated condensation (Cond.) summarizes the findings in Figure 3—figure supplement 1B, and the identified crucial regions are highlighted in green (low-complexity region, LCR4) and magenta (conserved domain, CD2). (B) Immunofluorescence of indicated HA-tagged proteins in transiently transfected HeLa cells. Scale bars: 20 µm. Insets are magnifications of the boxed areas. (C) Percentage of cells with condensates out of 1200 transfected cells from four independent experiments as in (B) (n=4). (D) Automated quantification of condensate number per nucleus by the Icy Spot Detector (Olivo-Marin, 2002) in 40 cells from four independent experiments as in (B) (n=40). (E) Relative luciferase activity reporting β-catenin-dependent transcription in HEK293T cells expressing the indicated constructs (n=4). (C–E), Results are mean ± SEM, *p<0.05, **p<0.01, ***p<0.001 (Student’s t-test).
-
Figure 3—source data 1
Excel file providing the numerical source data to Figure 3.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig3-data1-v1.xlsx

Mapping of dishevelled 2 (DVL2) regions promoting condensates and activity.
(A) Immunofluorescence of indicated HA-tagged proteins in transiently transfected U2OS cells. Scale bar: 20 µm. Insets are magnifications of the boxed areas. (B) Percentage of cells with condensates out of 900 transfected cells from three independent experiments as in (A) (n=3). (C) Relative luciferase activity reporting β-catenin-dependent transcription in HEK293T cells expressing the indicated constructs (ΔDEP n=9; ΔCD1, ΔCD2, ΔCFR n=5; ΔLCR1-4 n=4). (B, C) We categorized constructs with condensation above and below the dotted red line as condensate forming region (CFR) intact and CFR impaired, respectively, and this correlated consistently with Wnt pathway activation. (D) Example for automatic detection (red circles) of condensates formed by DVL2 ΔDEP in the nucleus (white signal) using the Icy Spot Detector (Olivo-Marin, 2002), as performed in Figure 3D. Note the very low number of false negatives (white condensate without red circle) and false positives (red circle without white condensate). Scale bar: 20 µm. (E, G) Area of the nuclei analyzed in Figure 3D (E) and in (F ,G) showing similar sizes of the investigated nuclei (n=40). (F) Automated quantification of condensate number per nucleus by the Icy Spot Detector in 40 cells from four independent experiments similarly performed as in Figure 3B but in U2OS cells (n=40). (B, C, E–G) Results are mean ± SEM, **p<0.01, ***p<0.001 (Student’s t-test).
-
Figure 3—figure supplement 1—source data 1
Excel file providing the numerical source data to Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig3-figsupp1-data1-v1.xlsx

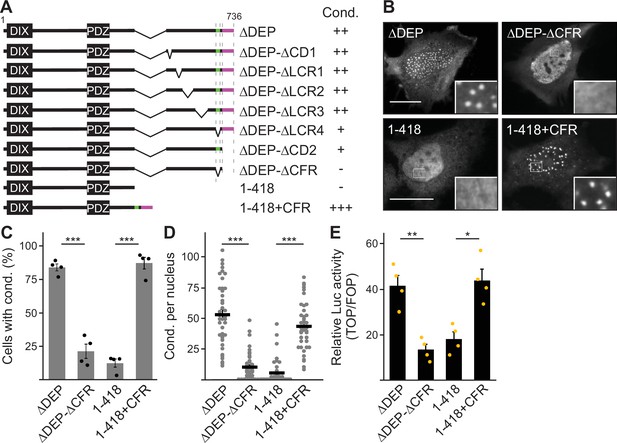
Low-complexity region (LCR4) and conserved domain (CD2) cooperate to promote DIX domain-dependent condensates.
(A, G) Immunofluorescence of indicated HA- (A and G upper row) or Flag-tagged proteins (G lower row) in transiently transfected U2OS cells. Scale bars: 20 µm. Insets are magnifications of the boxed areas. (B, E, F, H) Percentage of cells with condensates out of 1200 transfected cells from four independent experiments as in A, B, F, in Figure 3B (E) and in G ,H (n=4). For (E) (HeLa cells) and (F) (U2OS cells) only cells with cytosolic condensates were quantified. (C) Relative luciferase activity reporting β-catenin-dependent transcription in HEK293T cells expressing the indicated constructs (n=4). (B, C, E, F, H) Results are mean ± SEM, *p<0.05, **p<0.01, ***p<0.001 (Student’s t-test). (D) To scale schemes of used constructs with dishevelled 2 (DVL2) and AXIN1 parts depicted in black and gray, respectively. Indicated condensation (Cond.) refers to the findings in (B) and (H) and the identified crucial regions are highlighted in green (LCR4) and magenta (CD2).
-
Figure 3—figure supplement 2—source data 1
Excel file providing the numerical source data to Figure 3—figure supplement 2.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig3-figsupp2-data1-v1.xlsx
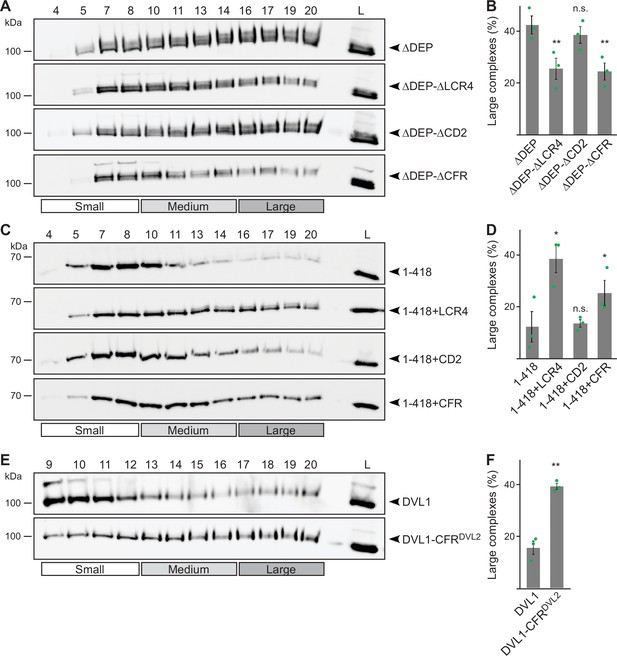
Low-complexity region (LCR4) mediates complex formation.
(A, C, E) Western blotting for indicated transiently expressed proteins bevor (L) and after fractionation (4-20) of HEK293T cell lysates via sucrose density ultracentrifugation. (B, D, F) Percentage of the protein that was engaged in large complexes as specified in A, C or E (n=3, refer to the legend to Figure 1D for more details). Results are mean ± SEM, *p<0.05, **p<0.01 (Student’s t-test).
-
Figure 4—source data 1
Excel file providing the numerical source data to Figure 4.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig4-data1-v1.xlsx
-
Figure 4—source data 2
PDF files containing the original, labeled blots to Figure 4.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig4-data2-v1.zip
-
Figure 4—source data 3
TIF files of the raw blots to Figure 4.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig4-data3-v1.zip
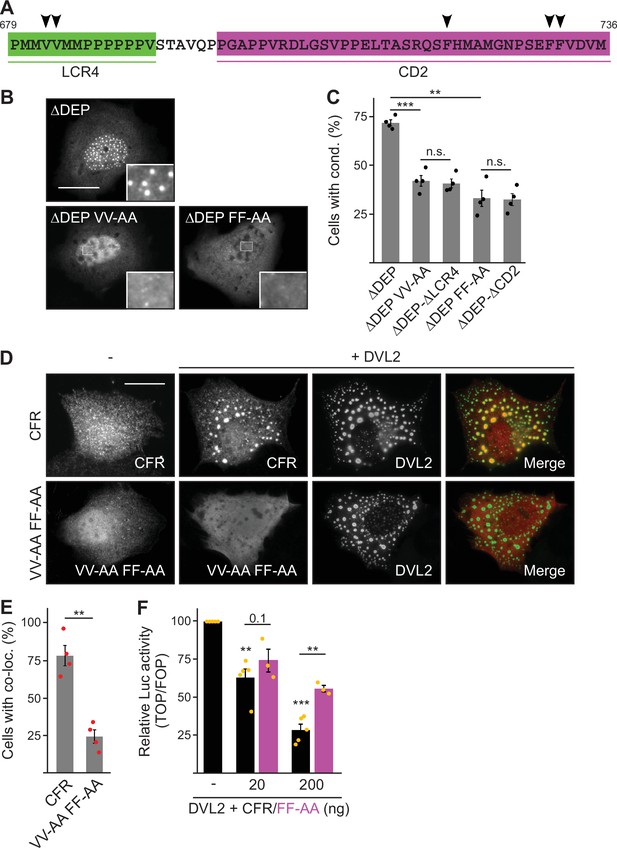
Low-complexity region (LCR4) and conserved domain (CD2) cooperatively mediate dishevelled 2 (DVL2)-DVL2 self-interaction.
(A) Condensate forming region (CFR) amino acid sequence with highlighted LCR4 (green) and CD2 (magenta). Arrowheads point to residues potentially mediating protein-protein interaction. (B) Immunofluorescence of indicated HA-tagged proteins in transiently transfected U2OS cells. Scale bar: 20 µm. Insets are magnifications of the boxed areas. (C) Percentage of cells with condensates out of 1200 transfected cells from four independent experiments as in (B) (n=4). (D) Immunofluorescence of indicated proteins in U2OS cells, which were transfected with Flag-CFR or the Flag-CFR VV-AA FF-AA mutant either alone or in combination with DVL2. Scale bar: 20 µm. (E) Percentage of cells exhibiting co-localization of CFR or CFR VV-AA FF-AA with DVL2 out of 1200 transfected cells from four independent experiments as in (D) (n=4). (F) Relative luciferase activity reporting β-catenin-dependent transcription in HEK293T cells transfected with DVL2 alone or together with rising amounts of either CFR or the CFR FF-AA mutant (black bars [CFR] n=5, magenta bars [CFR FF-AA] n=3). (C, E, F) Results are mean ± SEM, **p<0.01, ***p<0.001 (Student’s t-test).
-
Figure 5—source data 1
Excel file providing the numerical source data to Figure 5.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig5-data1-v1.xlsx

Specifying the residues mediating low-complexity region (LCR4) and conserved domain (CD2) function.
(A) Aggregation propensity (y-axis) of amino acid residues (x-axis) of wild-type (WT) (black curve) or valine residues (VV-AA) mutated (red curve) dishevelled 2 (DVL2) LCR4 according to the TANGO algorithm, which predicts aggregation nucleating regions (Fernandez-Escamilla et al., 2004). (B, E) Immunofluorescence of indicated HA-tagged proteins in transiently transfected U2OS cells. Scale bars: 20 µm. Insets are magnifications of the boxed areas. (C, F) Percentage of cells with condensates out of 1200 transfected cells from four independent experiments as in (B, C) and in (E, F) (n=4). (D, G, J) Relative luciferase activity reporting β-catenin-dependent transcription in HEK293T cells (D, G) and in T-REx cells with DVL1/2/3, RNF43, and ZNRF3 knockout (J upper panel, DVL tKO+) expressing the indicated constructs (n=4). (C, D, F, G, J) Results are mean ± SEM, *p<0.05, **p<0.01, ***p<0.001 (Student’s t-test). (H) Immunofluorescence of indicated proteins in U2OS cells, which were transfected with Flag-CFR together with either HA-DVL1 or HA-DVL3. Scale bar: 20 µm. (I, J, K) Western blotting showing the expression levels of endogenous DVL2 (-), transiently expressed HA-tagged DVL2 WT (DVL2) and indicated DVL2 mutants to Figure 7E (I) to the upper panel of (J) (J lower panel) and to Figure 7G (K) with α-tubulin serving as loading control. One out of three representative experiments is shown. Dotted vertical lines in (J) indicate the splicing of the blots.
-
Figure 5—figure supplement 1—source data 1
Excel file providing the numerical source data to Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig5-figsupp1-data1-v1.xlsx
-
Figure 5—figure supplement 1—source data 2
PDF files containing the original, labeled blots to Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig5-figsupp1-data2-v1.zip
-
Figure 5—figure supplement 1—source data 3
TIF files of the raw blots to Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig5-figsupp1-data3-v1.zip
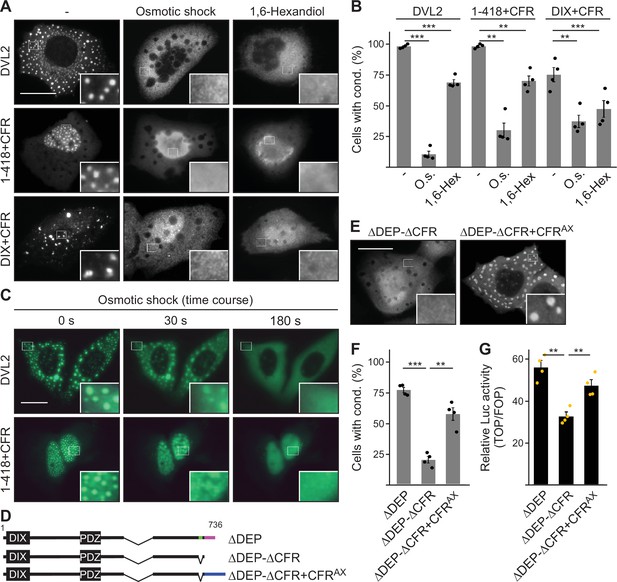
Condensate forming region (CFR)-induced condensates form via phase separation.
(A, E) Immunofluorescence of indicated HA-tagged proteins in transiently transfected U2OS cells, which were untreated, exposed to osmotic shock for 3 min, or treated with 1 µM 1,6-hexanediol for 1 hr. Scale bars: 20 µm. Insets are magnifications of the boxed areas. (B, F) Percentage of cells with condensates out of 1200 transfected cells from four independent experiments as in (A) or (E) (n=4). (C) Fluorescence of indicated GFP-tagged proteins in transiently transfected, alive U2OS cells at different time points of osmotic shock treatment. Scale bar: 20 µm. Insets are magnifications of the boxed areas. (D) To scale schemes of dishevelled 2 (DVL2) constructs. Low-complexity region (LCR4), CD2, and CFRAX are highlighted in green, magenta, and blue, respectively. (G) Relative luciferase activity reporting β-catenin-dependent transcription in HEK293T cells expressing the indicated constructs (n=4). (B, F, G) Results are mean ± SEM, **p<0.01, ***p<0.001 (Student’s t-test).
-
Figure 6—source data 1
Excel file providing the numerical source data to Figure 6.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig6-data1-v1.xlsx

Identifying a phase-separating region in AXIN1.
(A) Phase separating (condensate forming region, CFR) propensity score (y-axis) of amino acid residues of AXIN1 (x-axis) according to the catGRANULE algorithm, which predicts liquid-liquid phase separation propensity (Bolognesi et al., 2016). Red arrow points to a potential phase separating region in AXIN1 (CFRAX). (B) Left column: To scale schemes of artificial fusion proteins between the polymerizing AXIN1 DAX domain (gray) with CFRAX (different shades of blue). CFRAX was fused up to three times to the DAX domain (3xCFRAX-DAX). M3 indicates an inhibiting point mutation of DAX-mediated polymerization, which is illustrated by the red X (Fiedler et al., 2011). Middle column: Phase separating (CFR) propensity of the artificial AXIN1 proteins illustrated on the left according to the catGRANULE algorithm. The cumulative CFR scores of the proteins are shown in the upper right corner. Right column: Immunofluorescence of the Flag-tagged proteins indicated on the left in transiently transfected U2OS cells. Scale bar: 20 µm. (C) Percentage of cells with one to three (gray) or more than three condensates (black) out of 900 transfected cells from three independent experiments as in (B) (n=3). Results are mean ± SEM, **p<0.01, ***p<0.001 (Student’s t-test). The increase of condensates upon iterative fusion of CFRAX to the AXIN1 DAX domain validated its activity. Reduced condensation of 3xCFRAX-DAX M3 compared to 3xCFRAX-DAX demonstrated dependency of condensates on DAX-mediated polymerization, which we used as a specificity control for our artificial constructs, as it is the same for wild-type (WT) AXIN1 (Fiedler et al., 2011).
-
Figure 6—figure supplement 1—source data 1
Excel file providing the numerical source data to Figure 6—figure supplement 1.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig6-figsupp1-data1-v1.xlsx
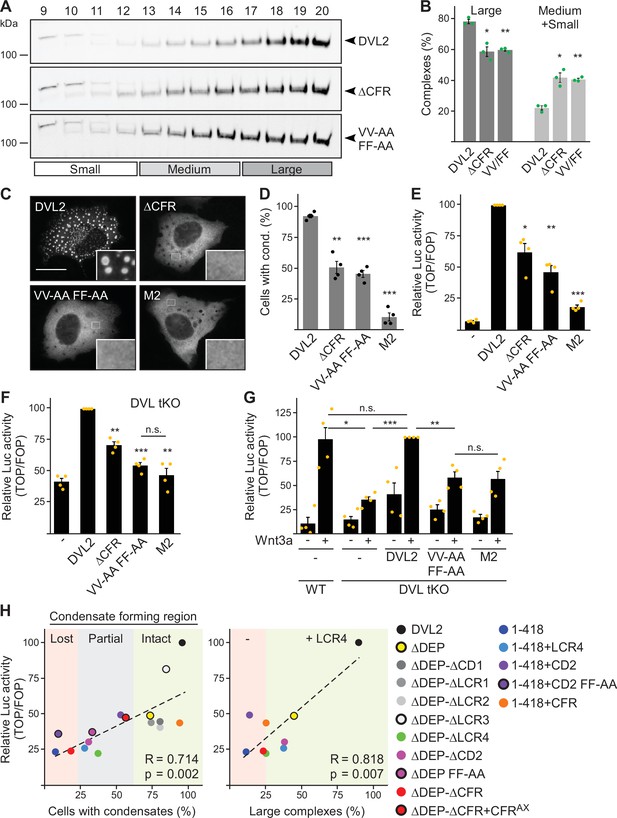
Dishevelled 2 (DVL2) condensate forming region (CFR) is crucial for Wnt signaling activity.
(A) Western blotting for indicated proteins, which were transiently expressed in HEK293T cells, after fractionation of cell lysates via sucrose density ultracentrifugation (see Figure 1E). (B) Percentage of the protein that was engaged in large complexes or in medium/small complexes, as specified in (A) (n=3, refer to the legend in Figure 1D for more details). (C) Immunofluorescence of indicated HA-tagged DVL2 proteins in transiently transfected U2OS cells. Scale bar: 20 µm. Insets are magnifications of the boxed areas. (D) Percentage of cells with condensates out of 1200 transfected cells from four independent experiments as in (C) (n=4). (E–G) Relative luciferase activity reporting β-catenin-dependent transcription in U2OS cells (E) in T-REx cells with DVL1/2/3 knockout (F DVL tKO) and in T-REx WT and DVL tKO cells (G), which were transiently transfected and treated with Wnt3a conditioned medium, as indicated (n=4). (B, D–G) Results are mean ± SEM, *p<0.05, **p<0.01, ***p<0.001 (Student’s t-test). (H) Plotting of Wnt pathway activation (y-axis) against either condensation (x-axis; left side) or complexation (x-axis; right side) for indicated DVL2 wild-type (WT) and mutant proteins. Correlation strength and significance are indicated by the Pearson‘s correlation coefficient R and the p-value, respectively. Note that condensation correlates with whether CFR is intact (low-complexity region, LCR4 and conserved domain, CD2 intact, green), partially intact (either LCR4 or CD2 intact, gray), or lost (neither LCR4 nor CD2 intact, red), and that the presence of LCR4 determines complexation. The plots summarize data that were shown before within this study.
-
Figure 7—source data 1
Excel file providing the numerical source data to Figure 7.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig7-data1-v1.xlsx
-
Figure 7—source data 2
PDF file containing the original, labelled blots to Figure 7.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig7-data2-v1.zip
-
Figure 7—source data 3
TIF files of the raw blots to Figure 7.
- https://cdn.elifesciences.org/articles/96841/elife-96841-fig7-data3-v1.zip
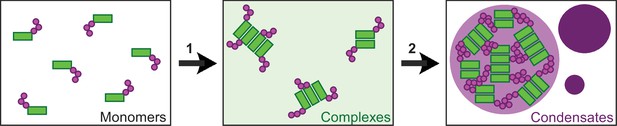
Two-step model of DVL2 condensate formation.
Schematic illustration of DVL2 domains mediating the formation of oligomeric complexes, such as LCR4 and the DEP domain (green rectangles) and of sticker domains mediating condensate formation, such as CD2 and the DIX domain (magenta circles). Oligomerization into complexes (1) (light green background) increases the valence of stickers in the complexes as compared to the monomers, which allows to overcome the low affinity of the isolated stickers and drives subsequent formation of condensates (2) (purple) by the multivalent stickers, according to the emerging stickers-model (Choi et al., 2020).
Videos
Osmotic shock dissolves dishevelled 2 (DVL2) condensates.
Fluorescence of GFP-DVL2 in transiently transfected, alive U2OS cells, which were imaged every 15 s over 3 min of osmotic shock treatment.
Osmotic shock dissolves condensate forming region (CFR)-induced condensates.
Fluorescence of GFP-1–418+CFR in transiently transfected, alive U2OS cells, which were imaged every 15 s over 3 min of osmotic shock treatment.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Homo sapiens) | DVL2 | GenBank | 1856 | |
| Cell line (Homo sapiens) | HEK293T | ATCC | CRL-3216 | |
| Cell line (Homo sapiens) | HeLa | ATCC | CCL-2 | |
| Cell line (Homo sapiens) | U2OS | ATCC | HTB-96 | |
| Cell line (Homo-sapiens) | T-REx | Paclíková et al., 2017 | ||
| Cell line (Homo-sapiens) | DVL tKO | Paclíková et al., 2017 | T-REx cells with DVL1/2/3 triple knockout | |
| Cell line (Homo-sapiens) | DVL tKO+ | Paclíková et al., 2021 | T-REx cells with DVL1/2/3, RNF43 and ZNRF3 penta knockout | |
| Antibody | anti-DVL2 (Rabbit polyclonal) | CellSignaling | Cat# 3216 S; | WB (1:1000) |
| Antibody | anti-DVL2 (Rabbit polyclonal) | CellSignaling | Cat# 3224 S | WB (1:1000) |
| Antibody | anti-AXIN1 (Rabbit polyclonal) | CellSignaling | Cat#: 2087 S | WB (1:1000) |
| Transfected construct (human) | siRNA to DVL2 | Dharmacon/Thermo Fisher Scientific; Soh and Trejo, 2011 | GGAAGAAAUUUCAGAUGAC | |
| Recombinant DNA reagent | HA-DVL2 (plasmid) | Bernkopf et al., 2015 | ||
| Recombinant DNA reagent | Expression plasmids for deleted and point mutated HA-tagged DVL2 | This paper | More than 25 plasmids have been newly generated (please see Molecular Biology for details) | |
| Chemical compound, drug | Sucrose | Fluka Analytical | 84097 | |
| Chemical compound, drug | Thyroglobulin (669 kDa) | Sigma Aldrich | T9145 | Size marker |
| Chemical compound, drug | Albumin (66 kDa) | Sigma Aldrich | A8531 | Size marker |
| Chemical compound, drug | 1,6-Hexanediol | Sigma Aldrich | 240117 | |
| Software, algorithm | TANGO algorithm | http://tango.crg.es/; Fernandez-Escamilla et al., 2004 | Prediction of aggregation sites | |
| Software, algorithm | Spot Detector tool, Icy bio-imaging software | Institut Pasteur, version 2.2.1.0; Olivo-Marin, 2002 | Quantify the numbers of condensates per cell | |
| Software, algorithm | catGRANULE algorithm | http://s.tartaglialab.com/update_submission/907235/f570b07a95; Bolognesi et al., 2016 | Prediction of liquid-liquid phase separation propensity | |
| Other | Wnt3a medium | Willert et al., 2003 | Wnt3a medium was prepared following the published protocol |





