Behavior: Prompting social investigation
Animals often need to assess whether a member of their species (a conspecific) that they have not met before will be a friend or a foe. As such, most adult animals would tend to investigate an unfamiliar peer over one which they were already acquainted with (Tapper and Molas, 2020).
Deciding whether and how to engage with an unknown individual relies on multiple levels of analysis underpinned by different brain networks and areas. First, the animal must identify that it has not encountered this specific peer before. For this, it must detect and check discrete features in the new conspecific against information deposited in memory networks after previous encounters. Certain regions of the hippocampus (the brain structure that helps to form memory and process emotions) have been implicated in this mechanism. Hippocampal neurons in the CA2 region and in the ventral portion of the CA1 area, for example, store social memories that allow animals to distinguish between new and familiar conspecifics (Hitti and Siegelbaum, 2014; Okuyama et al., 2016).
Once an unknown conspecific has been identified, other brain areas are then required to determine the appropriate course of action — whether to approach or retreat, for instance — and to prompt the associated behaviors. Emerging evidence indicates that the lateral septum may be involved in this process (Menon et al., 2022). This brain area – which is mostly formed of inhibitory neurons that repress the activity of the cells they project onto – is known to help shape social and emotional behaviors. The lateral septum receives projections from both the dorsal and ventral segments of the hippocampus and, in turn, connects with various regions involved in goal-directed behaviors. This includes the ventral tegmental area (or VTA; Rizzi-Wise and Wang, 2021). When dopaminergic neurons in this part of the brain are activated, such as during novel social interactions, they help drive the exploration of new stimuli and conspecifics (Gunaydin et al., 2014; Solié et al., 2022; Molas et al., 2024). Yet many of these pathways remain poorly understood. In particular, it is still unclear how the ventral hippocampus interacts with the lateral septum and the VTA to ‘transform’ social memories into motivations that promote individuals to investigate new conspecifics.
Now, in eLife, Malavika Murugan and colleagues at Emory University – including Maha Rashid as first author – report a new pathway between the ventral hippocampus, the lateral septum, and the VTA that regulates social novelty preference in mice (Figure 1; Rashid et al., 2024). To identify this circuit, the team carried out a social discrimination test which involved placing a mouse in an open chamber alongside two conspecifics of the same age and sex, which were caged on opposite sides of the apparatus. Only one of these individuals was known to the test subject, as they had been housed together for 72hours prior to the experiment. This is a much longer period than used in other protocols, allowing the animals to better recognize the features of the familiar peer.
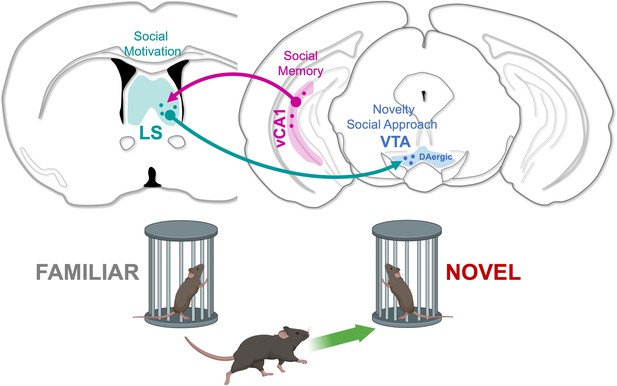
A neuronal circuit controlling social preferences.
vCA1 neurons (pink) in the ventral hippocampus encode social information and project to the lateral septum (LS; green), a region involved in social motivation. Lateral septum neurons establish monosynaptic connections onto dopaminergic neurons in the ventral tegmental area (VTA; blue), which promote exploration (green arrow) of new individuals.
© 2024, BioRender Inc. Figure 1 was created using BioRender, and is published under a CC BY-NC-ND license. Further reproductions must adhere to the terms of this license.
Preference for social novelty was determined by the amount of time the subject spent exploring the new individual relative to the familiar one. Without interventions, the mouse spent longer around the new conspecific.
Rashid et al. then used a technique known as chemogenetics to deactivate the neural pathway connecting the ventral hippocampus to the lateral septum, as this allowed them to assess whether these projections are required for animals to discriminate between novel and familiar social stimuli. The treatment did not affect the mice’s tendency to investigate new foods or objects more, but it disrupted their preference for social novelty (that is, the animals spent similar amounts of time investigating unknown and familiar individuals).
The team then used optogenetics to explore this effect in more detail, as this approach makes it possible to temporarily deactivate the pathway ‘at will’ as the animals perform the test. The experiments showed that the mice preferred investigating the conspecific that had been physically closest to them at the time their pathway had been silenced. Switching off the pathway promoted investigative behaviors towards a familiar individual (with the mice then having less time to spend exploring the unknown conspecific). In addition, if exposed to two new peers, the subjects explored the one which had been nearby during the manipulation. Overall, this suggests that preventing the activation of this pathway results in social investigations being more engaging. This led Rashid et al. to propose that the ventral hippocampus-lateral septum pathway may inhibit downstream regions which drive exploration of new social stimuli, such as the VTA.
The team therefore examined next whether the ventral hippocampus projects onto the pathway connecting the lateral septum to the VTA. To do so, they used monosynaptic rabies tracing, a method that helps reveal which neurons directly communicate with a specific cell. This allowed Rashid et al. to establish that the ventral hippocampus innervates cells in the lateral septum which connect to the VTA; disrupting the latter pathway with chemogenetic tools also prevented the preference for a novel mouse. Crucially, rabies tracing allowed Rashid et al. to show that neurons in the lateral septum directly project onto dopaminergic neurons in the VTA. In particular, the rostral part of the lateral septum, a subdivision recently implicated in the shift from novel to familiar social preferences in young mice, projected most strongly to the dopaminergic cells (de León Reyes et al., 2023).
Taken together, these results reveal a pathway connecting social memories stored in the ventral hippocampus to the centers responsible for motivational social behaviors (Figure 1). Ventral hippocampal cells connect to lateral septum neurons that are important for social behavior, which, in turn, project to VTA dopaminergic centers that control the animal’s social approach. Inhibition of the hippocampal-septal pathway disinhibits these VTA centers, resulting in the mouse being more interested in novel social interactions. These findings will aid in developing new therapies that improve social impairments in numerous neurodevelopmental and neuropsychiatric disorders.
References
-
Neurobiology of the lateral septum: regulation of social behaviorTrends in Neurosciences 45:27–40.https://doi.org/10.1016/j.tins.2021.10.010
-
Ventral CA1 neurons store social memoryScience 353:1536–1541.https://doi.org/10.1126/science.aaf7003
-
Midbrain circuits of novelty processingNeurobiology of Learning and Memory 176:107323.https://doi.org/10.1016/j.nlm.2020.107323
Article and author information
Author details
Publication history
Copyright
© 2024, Keppler and Molas
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 984
- views
-
- 51
- downloads
-
- 1
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Neuroscience
When holding visual information temporarily in working memory (WM), the neural representation of the memorandum is distributed across various cortical regions, including visual and frontal cortices. However, the role of stimulus representation in visual and frontal cortices during WM has been controversial. Here, we tested the hypothesis that stimulus representation persists in the frontal cortex to facilitate flexible control demands in WM. During functional MRI, participants flexibly switched between simple WM maintenance of visual stimulus or more complex rule-based categorization of maintained stimulus on a trial-by-trial basis. Our results demonstrated enhanced stimulus representation in the frontal cortex that tracked demands for active WM control and enhanced stimulus representation in the visual cortex that tracked demands for precise WM maintenance. This differential frontal stimulus representation traded off with the newly-generated category representation with varying control demands. Simulation using multi-module recurrent neural networks replicated human neural patterns when stimulus information was preserved for network readout. Altogether, these findings help reconcile the long-standing debate in WM research, and provide empirical and computational evidence that flexible stimulus representation in the frontal cortex during WM serves as a potential neural coding scheme to accommodate the ever-changing environment.
-
- Neuroscience
When navigating environments with changing rules, human brain circuits flexibly adapt how and where we retain information to help us achieve our immediate goals.






