Breast Cancer: How cell crowding causes cancer cells to spread
Cells must constantly respond and adapt to changes in their environment, such as temperature fluctuations and mechanical stress. These responses include cell growth and migration (Rice et al., 2020; Di et al., 2023). Cancer cells must also respond to their environment, in particular to the high density of nearby cells caused by their rapid proliferation. The mechanical pressure caused by this “cell crowding” can make the cancer cells more likely to spread and invade surrounding tissues, but we do not fully understand the mechanisms driving these processes. Understanding how cancer cells respond to cell crowding, and identifying the signals that make them more invasive in these conditions, is important for developing effective cancer treatments.
Ductal carcinoma in situ (or DCIS for short) is a non-invasive form of breast cancer that is confined to the milk ducts. Although it does not spread to surrounding tissue, if left untreated, it can transition into an invasive form of cancer that does spread (Pinder and Ellis, 2003). Now, in eLife, Inhee Chung and colleagues from George Washington University and Thomas Jefferson High School for Science and Technology – including Xiangning Bu as first author – report new insights into how cell crowding drives migration in certain types of DCIS cells (Bu et al., 2024).
First, Bu et al. demonstrated that DCIS cells categorized as more likely to be aggressive based on their appearance (also known as high-grade cells) become more invasive in crowded conditions. These invasive cells had a smaller volume than healthy cells or lower-grade DCIS cells. Cell volume changes are known to be closely linked to cell stiffness, which affects a cell’s ability to push through and invade surrounding tissues (Guo et al., 2017; Xie et al., 2024).
To investigate the mechanism behind this increased invasiveness, Bu et al. used a technique known as mass spectrometry to examine various proteins in the cells. In high-grade DCIS cells, they observed that cell crowding led to an increase in the number of various proteins on the plasma membrane of the cells. In particular, there was a striking 153-fold increase for a calcium ion channel called TRPV4. Transport proteins, such as ion channels and aquaporins, play a crucial role in the regulation of cell volume by creating osmotic gradients that cause water to move in and out of cells (Jentsch, 2016). TRPV4 is known to be activated by osmotic stress, mechanical stress, heat and certain chemical stimuli (Heller and O’Neil, 2007). This movement of TRPV4 and other ion-channel proteins from the cytoplasm to the plasma membrane, as well as reduced cell volume, could also be triggered by increasing osmotic pressure outside the cell, even in the absence of cell crowding.
Based on the finding that TRPV4 relocation was associated with cell crowding, Bu et al. next studied calcium levels in high-grade DCIS cells exposed to different cell crowding densities. This showed that cell crowding decreases intracellular calcium. Bu et al. next blocked TRPV4 function pharmacologically, finding that this mimicked the effects of cell crowding, promoting cell shrinkage and invasion. On the other hand, activating TRPV4 reversed these changes by increasing cell volume.
These results suggest that cell crowding acts as a mechanical cue that inhibits the action of TRPV4, reducing the transport of calcium ions into the cell and thus causing water to move out of the cell, leading to volume loss. This inhibition causes TRPV4 to move from the cytoplasm to the plasma membrane, to ensure that it is primed to become activated by mechanical stress later (Figure 1). Silencing the TRPV4 gene impaired the ability of cancer cells to respond to mechanical cues, leading to less calcium depletion, smaller volume changes, and reduced mobility.
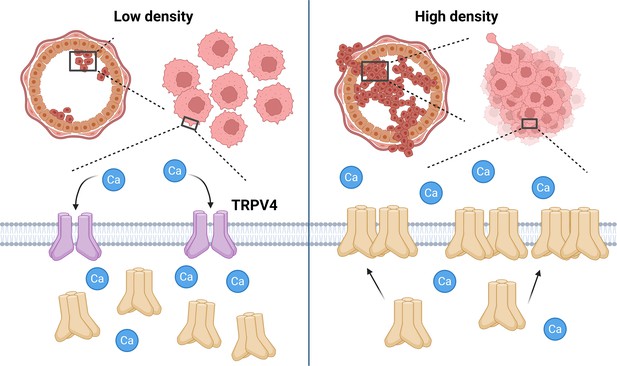
The influence of cell crowding on TRPV4 calcium ion channels.
When the density of breast cancer cells (pink) in the milk duct (large circular structure) is low (left), the majority of TRPV4 calcium ion channels are found in the cytoplasm of the cells in an inactive state (cream shapes), with a minority at the plasma membrane (gray) in an active state (purple shapes). The active channels facilitate the movement of calcium ions (Ca; blue circles) into the cell. However, when the density of cells is high (right), the mechanical stress caused by cell crowding inhibits TRPV4 activity and causes TRPV4 to move from the cytoplasm to the plasma membrane (cream shapes). This inhibition of TRPV4 decreases intracellular calcium levels, causing cell volume to shrink, which is a process associated with enhanced cellular invasiveness. The subsequent increase in TRPV4 at the plasma membrane also primes the cell for later activation of TRPV4 to compensate for the loss of calcium ions. These findings suggest that TRPV4 redistribution plays a critical role in the response of cancer cells to mechanical stress, potentially contributing to tumor progression. Created with BioRender.com.
Finally, analyzing patient-derived breast cancer tissue confirmed that TRPV4 is primarily localized to the plasma membrane in high-grade DCIS, but not in lower-grade DCIS or less aggressive cases. These findings suggest that TRPV4 plays a crucial role in influencing crowded cancer cells to become more invasive, as well as serving as a biomarker for identifying high-grade DCIS cells. This could open possibilities for targeting mechanosensitive pathways in cancer treatments and developing diagnostic tools for high-risk DCIS and other cancers.
While the findings of Bu et al. highlight a role for TRPV4 in invasiveness, other ion channels may also be involved (Ranade et al., 2015). Mass spectrometry screening revealed increases in two channel proteins – SCN11 and KCNN4 – caused by cell crowding. Additionally, an ion channel known as PIEZO1 was also found to relocate to the cell membrane under cell crowding conditions. Future research could explore how these channels interact and contribute to cancer adaptation. Other interesting questions include: why do different cell types respond differently to cell crowding, and what molecular mechanisms govern TRPV4 relocation? Addressing these questions could provide deeper insights into how ion channels contribute to cancer progression and help identify potential therapeutic targets.
References
-
Cellular mechanotransduction in health and diseases: from molecular mechanism to therapeutic targetsSignal Transduction and Targeted Therapy 8:282.https://doi.org/10.1038/s41392-023-01501-9
-
BookMolecular mechanisms of TRPV4 gatingIn: Liedtke WB, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton: CRC Press. pp. 113–124.https://doi.org/10.1201/9781420005844
-
VRACs and other ion channels and transporters in the regulation of cell volume and beyondNature Reviews Molecular Cell Biology 17:293–307.https://doi.org/10.1038/nrm.2016.29
-
Unveiling the intricate connection: cell volume as a key regulator of mechanotransductionAnnual Review of Biophysics 53:299–317.https://doi.org/10.1146/annurev-biophys-030822-035656
Article and author information
Author details
Publication history
Copyright
© 2025, Hua and Jiang
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1
- views
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Cancer Biology
- Genetics and Genomics
Tyrosine kinases play a crucial role in cell proliferation and survival and are extensively investigated as targets for cancer treatment. However, the efficacy of most tyrosine kinase inhibitors (TKIs) in cancer therapy is limited due to resistance. In this study, we identify a synergistic combination therapy involving TKIs for the treatment of triple negative breast cancer. By employing pairwise tyrosine kinase knockout CRISPR screens, we identify FYN and KDM4 as critical targets whose inhibition enhances the effectiveness of TKIs, such as NVP-ADW742 (IGF-1R inhibitor), gefitinib (EGFR inhibitor), and imatinib (ABL inhibitor) both in vitro and in vivo. Mechanistically, treatment with TKIs upregulates the transcription of KDM4, which in turn demethylates H3K9me3 at FYN enhancer for FYN transcription. This compensatory activation of FYN and KDM4 contributes to the resistance against TKIs. FYN expression is associated with therapy resistance and persistence by demonstrating its upregulation in various experimental models of drug-tolerant persisters and residual disease following targeted therapy, chemotherapy, and radiotherapy. Collectively, our study provides novel targets and mechanistic insights that can guide the development of effective combinatorial targeted therapies, thus maximizing the therapeutic benefits of TKIs.
-
- Cancer Biology
- Genetics and Genomics
Interpretation of variants identified during genetic testing is a significant clinical challenge. In this study, we developed a high-throughput CDKN2A functional assay and characterized all possible human CDKN2A missense variants. We found that 17.7% of all missense variants were functionally deleterious. We also used our functional classifications to assess the performance of in silico models that predict the effect of variants, including recently reported models based on machine learning. Notably, we found that all in silico models performed similarly when compared to our functional classifications with accuracies of 39.5–85.4%. Furthermore, while we found that functionally deleterious variants were enriched within ankyrin repeats, we did not identify any residues where all missense variants were functionally deleterious. Our functional classifications are a resource to aid the interpretation of CDKN2A variants and have important implications for the application of variant interpretation guidelines, particularly the use of in silico models for clinical variant interpretation.






