Cardiac Development: Getting to the heart of a good fossil
Like the famed Tin Man in The Wizard of Oz, if we palaeontologists ‘only had a heart’ we could do a lot more good science interpreting the significance of fossilised remains. For centuries, the fossil remains of ancient back-boned animals have been studied primarily from bones (skeletons) or traces (fossilised footprints). The possibility of finding preserved fossilised soft tissues was widely thought to be impossible as organic soft materials routinely decay in the process of burial and fossilisation.
However, certain fossil deposits, called Konservat Lagerstätten, are the result of rapid burial and special chemical conditions involving low-oxygen environments. These deposits can preserve or mineralogically “ghost” a range of soft tissues, even complete organs, in the fossilised organism.
Scientists have long known about the famous Burgess Shale fossils of soft-bodied worms and other invertebrate creatures buried by rapid mud slides around 508 million years ago. However, well-preserved fishes from the 113–119 million year old Santana Formation of Brazil were amongst the first vertebrate fossils to show evidence of preserved soft tissues. These include parts of the glandular stomachs and bands of muscles, with the original tissue having been mostly replaced by chemical processes (Martill, 1988; Wilby and Martill, 1992).
Finding whole preserved internal organs was a bit of a Holy Grail for palaeontology. Such discoveries can contribute a wealth of new anatomical information that is essential for understanding evolutionary patterns. Therefore the finding of a complete, well-preserved fossilised heart in an almost 120 million year old fish – now reported in eLife – is a major breakthrough for José Xavier-Neto of the Brazilian Biosciences National Laboratory, Vincent Fernandez of the European Synchotron Radiation Facility and colleagues from across Brazil and Sweden, including Lara Maldanis and Murilo Carvalho as joint first authors (Maldanis et al., 2016).
The new discovery was made by using synchrotron X-ray tomography to image fossils of the extinct fish Rhacolepis that were still entombed within limestone concretions. This technique images the fossil in thin sections; these images can then be processed to render the heart slice by slice and digitally restore the features of the organ. This method has been widely applied in palaeontology for the past decade or so to reveal many intricate soft tissue structures in fossils. These include the preserved brain of a 300 million year old fish from North America (Pradel et al., 2010) and a collection of superbly preserved soft tissues in 380 million year old fishes in Western Australia, such as an embryo with a mineralised umbilical cord (Long et al., 2008), nerve axial plate cells and muscle bundles (Trinajstic et al., 2007; 2013).
The Rhacolepis heart is the first example of a complete fossilised soft organ in an extinct fish, and the first fossilised heart in any fossil vertebrate (news reports from 2000 describing a dinosaur with a heart preserved have been recently disproven; Cleland et al., 2011). The heart shows excellent detail of the conus arteriosus, the conical extension of the ventricle that in certain fishes helps regulate blood outflow via the valves in the conus. It also shows the pattern of five rows of valves inside it. The anatomical interpretation of Maldanis, Carvalho et al. is further reinforced by detailed comparisons with dissected hearts from closely-related living fishes called tarpons, which show similar structures in the same relative positions.
The discovery of the fossilised Rhacolepis heart is significant because the range of valve patterns in early ray-finned fish hearts is strikingly diverse. Some, like the very primitive (“basal”) ray-finned fish Polypterus (the African reedfish), have nine rows of valves, whereas most of the modern group of ray-fins, the teleosts, have just a single outflow valve in the heart.
In between these two groups of fishes we can now enter Rhacolepis, a fish belonging to an extinct group that has a basal position amongst the teleosts (Arratia, 2010; Figure 1). Thus the five rows of valves shown by the fossil seems to represent a good intermediate condition between the most primitive pattern and the most advanced type. However, as we well know, in biology simple patterns often hold more complex underlying meanings. For example, the valve pattern within the conus arteriosus has simplified independently in sturgeons and bowfins. There is also evidence for an independent increase in the numbers of valves in some basal ray-finned fishes (Lepisosteiformes, Polypteriformes), so interpreting evolutionary patterns from one data point is always risky business. Maldanis, Carvalho et al. claim that the Rhacolepis heart supports a possible case of gradual speciation (as opposed to drastic anatomical changes from one generation to the next). With only one data point, this hypothesis cannot be tested further.
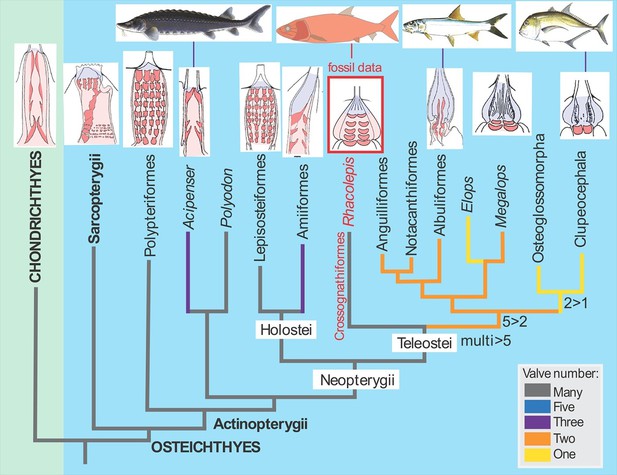
The fossil heart data from Rhacolepis shows an intermediate condition between the many-valved types seen in basal ray-finned fishes (actinopterygians) like Polypteriformes (the order that includes the African reedfish) and the single-valved hearts in modern teleosts.
Images of the hearts showing valves (red) are oriented with the top of the image pointing towards the head of the fish (taken from Maldanis et al., 2016).
Nonetheless, for the first time we actually do have a data point to study the detailed anatomy of a fossilised heart in an extinct group of fishes. The find demonstrates the immense potential for more discoveries of this nature, enabling more discussion of the comparative anatomy of soft organs in extinct animals. With more highly detailed discoveries like this one, I confidently predict we will one day be able to really get to the heart of resolving the mysteries of early vertebrate evolution. That day is not far away.
References
-
The Clupeocephala re-visited: Analysis of characters and homologiesRevista De Biología Marina Y Oceanografía 45:635–657.https://doi.org/10.4067/S0718-19572010000400009
-
Preservation of fish in the Cretaceous Santana Formation of BrazilPalaeontology 31:1–18.
-
Skull and brain of a 300-million-year-old chimaeroid fish revealed by synchrotron holotomographyProceedings of the National Academy of Sciences of the United States of America 106:5224–5228.https://doi.org/10.1073/pnas.0807047106
Article and author information
Author details
Publication history
Copyright
© 2016, Long
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,725
- views
-
- 172
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Ecology
Global change is causing unprecedented degradation of the Earth’s biological systems and thus undermining human prosperity. Past practices have focused either on monitoring biodiversity decline or mitigating ecosystem services degradation. Missing, but critically needed, are management approaches that monitor and restore species interaction networks, thus bridging existing practices. Our overall aim here is to lay the foundations of a framework for developing network management, defined here as the study, monitoring, and management of species interaction networks. We review theory and empirical evidence demonstrating the importance of species interaction networks for the provisioning of ecosystem services, how human impacts on those networks lead to network rewiring that underlies ecosystem service degradation, and then turn to case studies showing how network management has effectively mitigated such effects or aided in network restoration. We also examine how emerging technologies for data acquisition and analysis are providing new opportunities for monitoring species interactions and discuss the opportunities and challenges of developing effective network management. In summary, we propose that network management provides key mechanistic knowledge on ecosystem degradation that links species- to ecosystem-level responses to global change, and that emerging technological tools offer the opportunity to accelerate its widespread adoption.
-
- Ecology
- Evolutionary Biology
Eurasia has undergone substantial tectonic, geological, and climatic changes throughout the Cenozoic, primarily associated with tectonic plate collisions and a global cooling trend. The evolution of present-day biodiversity unfolded in this dynamic environment, characterised by intricate interactions of abiotic factors. However, comprehensive, large-scale reconstructions illustrating the extent of these influences are lacking. We reconstructed the evolutionary history of the freshwater fish family Nemacheilidae across Eurasia and spanning most of the Cenozoic on the base of 471 specimens representing 279 species and 37 genera plus outgroup samples. Molecular phylogeny using six genes uncovered six major clades within the family, along with numerous unresolved taxonomic issues. Dating of cladogenetic events and ancestral range estimation traced the origin of Nemacheilidae to Indochina around 48 mya. Subsequently, one branch of Nemacheilidae colonised eastern, central, and northern Asia, as well as Europe, while another branch expanded into the Burmese region, the Indian subcontinent, the Near East, and northeast Africa. These expansions were facilitated by tectonic connections, favourable climatic conditions, and orogenic processes. Conversely, aridification emerged as the primary cause of extinction events. Our study marks the first comprehensive reconstruction of the evolution of Eurasian freshwater biodiversity on a continental scale and across deep geological time.





