Computer-guided design of optimal microbial consortia for immune system modulation
Abstract
Manipulation of the gut microbiota holds great promise for the treatment of diseases. However, a major challenge is the identification of therapeutically potent microbial consortia that colonize the host effectively while maximizing immunologic outcome. Here, we propose a novel workflow to select optimal immune-inducing consortia from microbiome compositicon and immune effectors measurements. Using published and newly generated microbial and regulatory T-cell (Treg) data from germ-free mice, we estimate the contributions of twelve Clostridia strains with known immune-modulating effect to Treg induction. Combining this with a longitudinal data-constrained ecological model, we predict the ability of every attainable and ecologically stable subconsortium in promoting Treg activation and rank them by the Treg Induction Score (TrIS). Experimental validation of selected consortia indicates a strong and statistically significant correlation between predicted TrIS and measured Treg. We argue that computational indexes, such as the TrIS, are valuable tools for the systematic selection of immune-modulating bacteriotherapeutics.
https://doi.org/10.7554/eLife.30916.001eLife digest
The role of the immune system is to protect the body from infection. It does this by using a powerful toolkit that isolates pathogens and removes damaged tissue. When directed against bacteria and viruses, the system helps to keep the body safe, but an imbalance of the components of the immune system can lead to inflammatory or allergic diseases.
The body has built-in mechanisms to shut off the immune response. For example, regulatory T-cells are immune cells with an anti-inflammatory effect, meaning they can switch off inflammation after the immune system has cleared an invasion. If their numbers are too low, it can contribute to unwanted inflammation. In ulcerative colitis, for instance, an unbalanced immune system mistakenly attacks healthy tissue. This causes inflammation and ulcers in the intestine, resulting in bleeding and weight loss.
Previous studies revealed that bacteria in the gut might help to control regulatory T-cell numbers. Certain combinations of bacteria can stimulate these regulatory T-cells and potentially dampen inflammation. Tweaking the different populations of microbes in the gut could provide a new way to treat diseases like ulcerative colitis. But, identifying the best combination to use is a major challenge. Testing them all one by one would be extremely challenging. Now, Stein, Tanoue et al. present a mathematical model designed to predict the best microbe-mix to use.
The model incorporated data from regulatory T-cells and microbes gathered from mice to estimate the contribution that different strains of bacteria make to regulatory T-cell numbers. This then fed into an ecological model predicted how different combinations of bacteria would behave in mice. The model aimed to fulfill two key criteria. First, to find combinations that can form stable, long-term bacterial colonies alone and when faced with competing microbes. Second, to boost regulatory T-cell numbers, helping them to expand to correct the immune imbalance.
To test the predictions, mice received combinations of bacteria suggested by the model. The model had predicted some combinations to be 'weak' at inducing regulatory T-cells, some 'intermediate' and some 'strong'. The results matched the predictions, validating the method.
This new model allows the rapid design of microbes that could dampen the immune response in the gut and paves the way for new treatments to correct imbalances of the immune system. By using already available data, it should be possible to expand the model to other diseases with imbalance in the immune system. In future, similar models could also find combinations for other medical uses. For example, to optimise the delivery of cancer immunotherapy, or to modulate the immune system after an organ transplant.
https://doi.org/10.7554/eLife.30916.002Introduction
The intestinal microbiota has been shown to critically influence a multitude of host physiological functions, often through modulation of the immune system (Gevers et al., 2014; Paun et al., 2016). Evidence includes studies in germ-free mice, which show that both pro-inflammatory T-helper 17 (Th17) cells and anti-inflammatory regulatory T-cells (Treg) are reduced in numbers in the intestinal lamina propria relative to conventional or specific pathogen-free mice (Atarashi et al., 2008), and that repopulation with specific bacteria can reconstitute them (Atarashi et al., 2015; Ivanov et al., 2009). One route to how the microbiota influences immune system activation is by the production of small molecules (Donia and Fischbach, 2015). Microbially produced short-chain fatty acids (SCFAs) were shown to facilitate extrathymic differentiation of immune system modulatory Treg (Arpaia et al., 2013; Atarashi et al., 2013; Smith et al., 2013) and are implicated in Treg-dependent anti-inflammatory properties of a mix of 17 human-derived Clostridia strains (Atarashi et al., 2013; Tanoue et al., 2016). Interestingly, individual Clostridia strains only have a modest effect on Treg induction – optimal induction relies on the synergistic interplay of several strains (Atarashi et al., 2013; Belkaid and Hand, 2014). Because of these properties, there is currently great interest in the manipulation of this community for the treatment of inflammatory and allergic diseases (Honda and Littman, 2012; Olle, 2013; Vieira et al., 2016). Efforts involving transplantation of bacteria from healthy humans to humans with C. difficile infections or with metabolic diseases have provided evidence that microbiota repopulation could be used as possible strategy for disease prevention and treatment (van Nood et al., 2013; Vrieze et al., 2012). For this reason, intestinal supplementation of defined compositions of gut bacteria to treat a range of diseases is currently being pursued by multiple bio-pharmaceutical companies (Garber, 2015). Determining what microbial consortia can colonize the host and stably coexist with an already resident microbial community, while inducing the desired immune response, is still a major challenge that hinders the translation of these efforts into the clinic (Maldonado-Gómez et al., 2016; Weil and Hohmann, 2015).
In previous work, some of us identified a set of 17 potent Treg-inducing Clostridia strains to select candidate subsets from (Atarashi et al., 2013). The determination of maximally immune-phenotype inducing combinations from these 17 strains is, however, experimentally infeasible as it would require the testing of 217–1 = 131071 possible subsets in mice (Faith et al., 2014). Therefore, a computational approach to prioritize which subsets to validate experimentally would have great utility. To our knowledge, to date there has been no model that allows for the simultaneous prediction of the dynamics of both the microbiota and the host immune response. Building on the approach to select for optimal bacterial combinations from (Faith et al., 2014) and other efforts aimed at identifying potential immune-modulating microbes (Geva-Zatorsky et al., 2017; Schirmer et al., 2016), we overcome this problem by proposing a mathematical modeling-based framework that, by resolving microbiome–immune system interactions with parameters constrained to experimental observations of microbiome and immune effectors, allows computational optimization of immune-stimulating bacterial combinations. To achieve this, we use a series of logically connected analyses that capture CD4+FOXP3+ Treg accumulation in the colonic lamina propria (Omenetti and Pizarro, 2015; Round and Mazmanian, 2010) in response to the dynamics of human-derived Clostridia strains in germ-free mice, which had previously been shown to potently induce Treg expansion (Atarashi et al., 2013) (Figure 1). To constrain this proposed model framework to experimental data, we combine previously published (Atarashi et al., 2013) and newly generated fluorescence-activated cell scanning (FACS) data with simulated and newly generated time-series colonization data from germ-free mice (Figure 2A). Subsequently, by applying a microbiome–Treg mathematical model to this combined data set, we infer each strain’s individual contribution to the CD4+FOXP3+ Treg pool (Figure 2B) and obtain an estimate of every possible consortium’s Treg induction potential. To justify the usage of predicted mono-colonization concentrations from a previously developed microbiome ecological model (Bucci et al., 2016), we validate its ability in predicting temporal dynamics in response to different subsets of Treg-inducing strains (Figure 3A) and quantify the deviation of data and predictions (Figure 3B). Introduction of the TrIS, which assigns a score to every predicted steady-state microbial composition, enables us to identify combinations that robustly maximize Treg induction (Figure 4A). We analyze the relationship of TrIS and biomass (Figure 4B) as well as metabolic features of the consortia (Figure 4C and D). Most importantly, we demonstrate the utility of our approach in predicting the potency of selected microbial combinations by validating the Treg induction and colonization ability of model-predicted strong, intermediate and weak Treg-inducing consortia in vivo (Figure 4E).
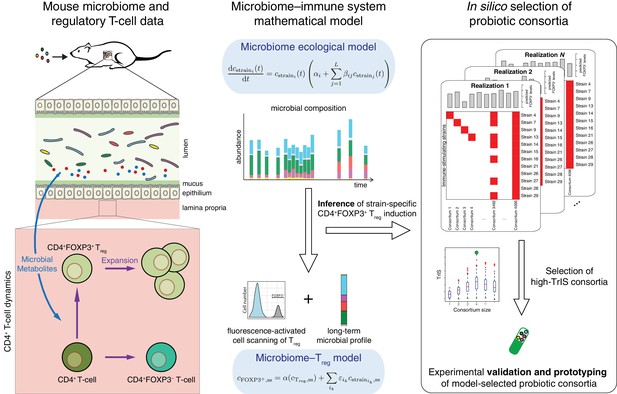
Conceptual figure.
A microbiome–immune system mathematical model describing the activation of regulatory T-cells (Treg) in response to colonization profiles of Treg-stimulating Clostridia strains is the central element of this work. It consists of a previously derived microbiome ecological model that describes the short and long-term temporal dynamics of Clostridia strains in germ-free mice (Bucci et al., 2016) and is supplemented by a microbiome–Treg model of CD4+FOXP3+ Treg activation in response to long-term compositions in the microbiome. The individual contribution of each strain to the measured Treg activation is inferred using long-term colonization data from mouse experiments with subsets of these strains and corresponding measurements of Treg induction. The Treg-induction score, TrIS, which accounts for ecological stability and immune activation, assigns a score to every possible strain combination and thereby identifies candidate probiotic consortia for experimental validation.
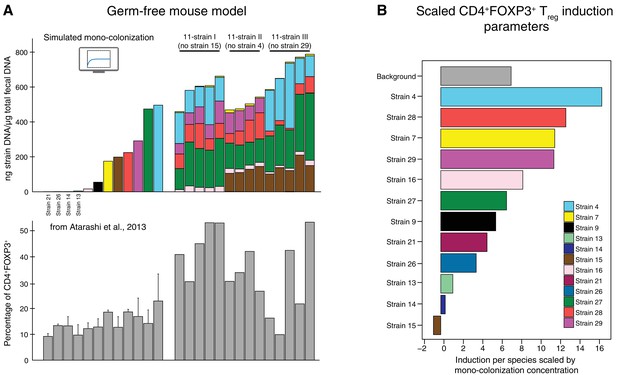
Data used for inference of the CD4+FOXP3+Treg induction parameters.
(A) To infer the strain-resolved CD4+FOXP3+ Treg induction parameters CD4+FOXP3+ Treg abundance measurements and corresponding microbiome colonization data are used. CD4+FOXP3+ Treg data of single strains originates from previously published measurements from Atarashi et al. (2013). Because in Atarashi et al. (2013) microbial mono-colonization levels were not measured, a previously published predictive model (Bucci et al., 2016) was used to simulate these experiments. In addition, newly generated CD4+FOXP3+ Treg and colonization mouse stool data from three 11-strain combinations was included into the analysis (see also Figure 3). The 11-strain combinations were chosen according to results of the ‘keystoneness’ analysis previously described in Bucci et al. (2016). The microbiome compositions of these three 11-strain combinations were estimated by strain-specific qPCR. (B) The resulting CD4+FOXP3+ Treg induction parameters quantify the contribution of each individual strain to Treg induction. (Coefficients are scaled by the microbial mono-colonization concentrations for display reasons.)
-
Figure 2—source data 1
Data containing the microbiome compositions, the corresponding measured CD4+FOXP3+ proportions and the derived induction parameters.
- https://doi.org/10.7554/eLife.30916.005
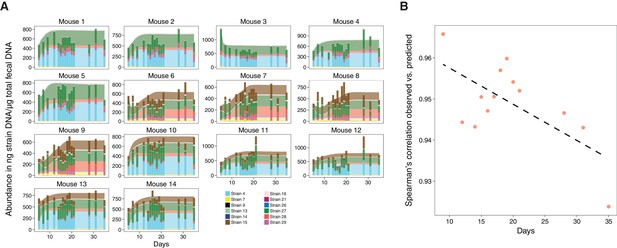
Validation of microbiome model describing the dynamics of Clostridia strains in germ-free mice.
(A) The model-predicted dynamics of three 11-strain combinations from the 13 Clostridia strains described in Bucci et al. (2016) (areas) are compared to measured data from germ-free mice (stacked bars) and each panel corresponds to an individual mouse (Figure 2A). Predictions were obtained by numerically integrating the corresponding generalized Lotka–Volterra equations with parameters from Bucci et al. (2016) using only each mouse’s initial microbial composition. In timelines/mouse 1–5, Strain 15 is absent referring to 11-strain set I (five biological replicates); in timelines 6–9, Strain 4 is absent (11-strain set II; four biological replicates), and in timelines 10–14, Strain 29 is absent (11-strain set III; five biological replicates). Data were obtained by qPCR of genes specific to each strain. Densities are computed as averages of three technical replicates. The number of mice used in each condition was chosen consistently with previous experimental work (Atarashi et al., 2013; Bucci et al., 2016) and combined with extensive in silico testing of the inference error as a function of sampling frequency and noise levels (Bucci et al., 2016). (B) Spearman’s rank correlation coefficient between observed and predicted data was computed at different time points. All displayed coefficients have a p-value of less than 10−16.
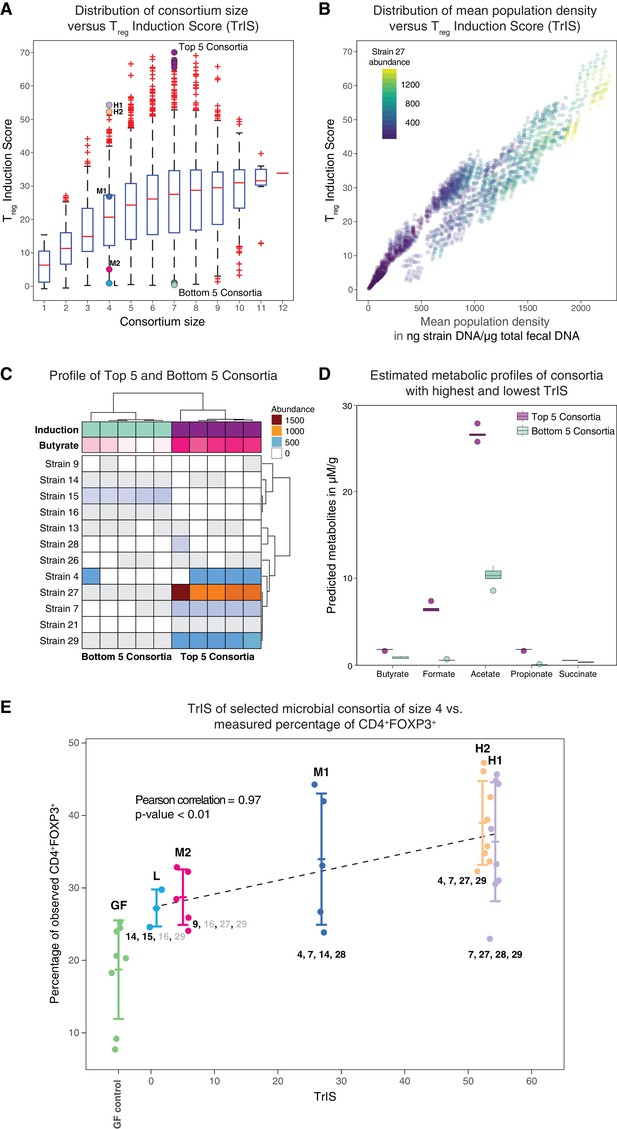
Identification and experimental validation of model-predicted Treg-stimulating consortia.
(A) Predicted Treg Induction Score (TrIS) in germ-free mice as a function of probiotic consortium size. Each data point represents one of the 2N−1 possible non-trivial consortia of size N. The five highest and lowest TrIS consortia of size seven are highlighted by violet and cyan filled dots, respectively, as well as, the 4-strain consortia used for experimental validation in subpanel E. (B) Distribution of the TrIS plotted against the total population density of each model-predicted consortium. This analysis shows a strong correlation (Spearman’s Rank Correlation with coefficient 0.88 and p<0.05) between TrIS and total microbial density. The color of each dot represents the abundance of Strain 27, which is predicted to strongly colonize in high-TrIS subsets. (C) Heatmap of consortium membership of the five highest and lowest TrIS-consortia of size seven and their predicted Treg induction and estimated butyrate production using data from Atarashi et al. (2013). (D) Estimation of potential SCFA output based on single-strain in vivo metabolic profiling from Atarashi et al. (2013). The five highest TrIS consortia of size seven (from 4C) are predicted to have a significantly higher SCFA output relative to the five lowest ones. (E) Five 4-strain consortia were used for experimental validation: the two highest-ranked consortia (H1 and H2, respectively), the lowest-ranked consortium (L) and two intermediates (M1, M2). M1 and M2 were included because of interest in other disease areas. The experimentally introduced strains are listed next to each bar/consortium. Strains with numbers in black were detected by 16S rRNA sequencing in mouse stool samples, strains with numbers in gray were introduced but failed to colonize. A Pearson’s correlation of 0.97 between the predicted TrIS and the average of each consortium’s measured CD4+FOXP3+ Treg percentage proves the ability of the TrIS-based selection to correctly recover the experimentally observed Treg induction. (Pearson’s correlation of all points has a value of 0.54.) Importantly, the H2 consortium displays an average increase in immune activity of 107% relative to the average germ-free mouse control. Eight biological replicates were used for GF, H1 and H2, five biological replicates were used for M1, M2 and three biological replicates were used for L. Replication and design of the validation experiment for Treg induction assessment is consistent with work from us and others (Atarashi et al., 2013; Narushima et al., 2014).
-
Figure 4—source data 1
TrIS, TrIS rank and predicted median steady-state concentrations of the selected microbial consortia from Figure 4E.
- https://doi.org/10.7554/eLife.30916.010
We envision that our framework, while in this study tailored to finding combinations ameliorating auto-inflammatory conditions, may also have direct relevance to other immune-system enhancing applications such as the optimal delivery of probiotic-based cancer immunotherapies (Garrett, 2015).
Results
Generation of multimodal microbiome–Treg data set
The goal of this study is to develop a mathematical modeling-based framework to rapidly and systematically select microbial consortia that maximize a desired immune outcome when introduced in a specific host microbial background. To achieve this, we combine (i) a microbiome ecological model that accurately describes the dynamics of these bacteria in the host with (ii) a microbiome–Treg mathematical model that characterizes the contribution of every strain to the immune phenotype of interest given corresponding microbiome colonization data (Figure 1). For (i), we gathered newly produced quantitative polymerase chain reaction (qPCR) colonization data from gnotobiotic mice and combined it with a previously published microbiome ecological model of the dynamics of 12 Treg-inducing Clostridia strains that are part of an original consortium of 17 Treg-inducing strains discovered by some of us (Atarashi et al., 2013) (see also Table 1 for a breakdown of strains used in each study). In contrast to the original study of (Bucci et al., 2016), we included only 12 of the 13 strains used there because, based on the modeling and analysis of Bucci et al. (2016), Strain 6 from the 13-strain set was predicted to not stably colonize in the presence of the other 12 strains. The published colonization data have been reported in our previous work (Bucci et al., 2016) and include time-series measurements of microbial abundances by qPCR under dietary perturbations. These are used to derive a predictive microbiome ecology model in gnotobiotic conditions based on an extension of the generalized Lotka–Volterra (gLV) equations (Hofbauer and Sigmund, 1998) as introduced in Stein et al. (2013). For the newly generated dataset, we gavaged 14 mice with one of three possible 11-strain subsets from the 12-strain subset of original 13 strains (Figure 2) and used the derived stool measurements to validate the ability of our mathematical model in predicting unseen conditions. The three 11-strain subsets were chosen based on our ‘keystoneness’ definition, a measure describing the marginal predicted quantitative effect of removing each strain from the full community (Bucci et al., 2016). Specifically, we included two 11-strain combinations each missing one of the two highest keystoneness-scoring strains (VE202 Strain 15 and VE202 Strain 4) and one 11-strain combination which lacks the lowest keystoneness scoring strain (VE202 Strain 29). In analogy to Bucci et al. (2016), each strain’s density was profiled over time by qPCR with strain-specific primers (see Materials and methods). To resolve the contribution of each of these to Treg induction for point (ii) we coupled the colonization data from fecal content with newly collected and published FACS measurements of the CD4+FOXP3+ Treg population in the lamina propria of these mice (Figure 2A). As it is crucial to capture each strain’s contribution alone and in combination with others, we also included CD4+FOXP3+ Treg measurements from our previously published mono-colonization experiments (Atarashi et al., 2013) (Figure 2A). Due to the fact that the single-strain mono-colonization concentrations were not measured in these experiments (Atarashi et al., 2013), we simulated them using the microbiome ecological model and parameters from (Bucci et al., 2016). This choice was supported by the model's capability in predicting unseen validation data (Figure 3A). Spearman’s rank-order correlation coefficient ranges from 0.92 to 0.98 (p-value<10−16) between observations and predictions depending on the time point (Figure 3B).
List of strains used with respect to our previous published work.
The original publication (Atarashi et al., 2013) reported on 17 immune-modulating bacteria. In Bucci et al. (2016), a dynamical ecological model for 13 of the original 17 strains was presented. These 13 strains were selected because they do not harbor antibiotic resistance genes. For our study, we used a 12-strain subset of these 13 strains based on ecological stability considerations. Colonization and CD4+FOXP3+Treg data for the three 11-strain sets, measured by qPCR and FACS, were used along with the simulated mono-colonization concentrations (Figure 2) and included into the microbiome–immune system model (Figure 1). The resulting parameters were employed to predict ecologically stable subsets with different Treg induction potentials. 4-strain subsets were selected to validate the predictions of the mathematical model (see main text and Figure 3). Strain 1: Clostridium saccharogumia/Clostridium ramosum JCM1298, Strain 3: Flavonibacter plautii/Pseudoflavonifractor capillosus, Strain 4: Clostridium hathewayi/Clostridium saccharolyticum WM1, Strain 6: Blautia coccoides/Lachnospiraceae bacterium, Strain 7: Clostridium bolteae, Strain 8: Clostridium sp. MLG055/Erysipelotrichaceae bacterium 2 44A, Strain 9: Clostridium indolis/Anaerostipes caccae DSM 14662, Strain 13: Anaerotruncus colihominis, Strain 14: Ruminococcus sp. ID8/Lachnospiraceae bacterium 2 1 46FAA, Strain 15: Clostridium asparagiforme/Clostridium lavalense, Strain 16: Clostridium symbiosum, Strain 18: Clostridium ramosum, Strain 21: Eubacterium fissicatena/Eubacterium contortum/Clostridium sp. D5, Strain 26: Clostridium scindens/Lachnospiraceae bacterium 5 1 57FAA, Strain 27: Lacnospiraceae bacterium A4/Lachno bacterium 3 1 57FAA CT1, Strain 28: Clostridium sp. 316002/08, Strain 29: Lacnospiraceae bacterium A4/Lachno bacterium 3 1 57FAA CT1.
| Atarashi et al., 2013 | Bucci et al., 2016 | 11-str. I | 11-str. II | 11-str. III | H1 | H2 | M1 | M2 | L | |
|---|---|---|---|---|---|---|---|---|---|---|
| Strain 1 | x | |||||||||
| Strain 3 | x | |||||||||
| Strain 4 | x | x | x | x | x | x | ||||
| Strain 6 | x | x | ||||||||
| Strain 7 | x | x | x | x | x | x | x | x | ||
| Strain 8 | x | |||||||||
| Strain 9 | x | x | x | x | x | x | ||||
| Strain 13 | x | x | x | x | x | |||||
| Strain 14 | x | x | x | x | x | x | x | |||
| Strain 15 | x | x | x | x | x | |||||
| Strain 16 | x | x | x | x | x | x | x | |||
| Strain 18 | x | |||||||||
| Strain 21 | x | x | x | x | x | |||||
| Strain 26 | x | x | x | x | x | |||||
| Strain 27 | x | x | x | x | x | x | x | x | ||
| Strain 28 | x | x | x | x | x | x | x | |||
| Strain 29 | x | x | x | x | x | x | x | x |
Derivation of the microbiome–Treg mathematical model
We used the described microbiome colonization data and corresponding CD4+FOXP3+ Treg measurements to determine the contribution of each strain to the Treg pool. We begin by subdividing the population of CD4+ T-cells into two major subpopulations depending on their intracellular FOXP3 expression: CD4+FOXP3+ Treg and the remainder among the CD4+ T-cells, the conventional CD4+FOXP3− T-cells (Bilate and Lafaille, 2012; Rudensky, 2011). The concentration of CD4+ T-cells at time in the colonic lamina propria, , is then the combination of these two T-cell populations, . To include a variety of effects into our model, we assume T-cell dynamics to follow the gLV equations (Gerber, 2014; Hofbauer and Sigmund, 1998), which also account for the effect of the microbial strains in the lumen on the CD4+FOXP3− T-cell subpopulation. In addition, we use an extension of the standard gLV equations to include the impact of the Clostridia strains in terms of external perturbations (Stein et al., 2013). The resulting microbiome–Treg mathematical model is found as,
where denotes the basal growth rate and the self-interaction term of the CD4+FOXP3+ Treg population. The interaction terms and represent the effect of the CD4+FOXP3− T-cell on the CD4+FOXP3+ Treg population and of the CD4+FOXP3+ Treg on the CD4+FOXP3− T-cell population, respectively (d’Onofrio, 2005). Consequently, positive interaction parameters correspond to activation, negative ones to inhibition. Moreover, denotes the effect of strain on the CD4+FOXP3+ Treg population. For long-term observations, , the dynamics of are given by its (non-trivial) steady-state solution, which simplifies the then static microbiome–Treg mathematical model of the relative CD4+FOXP3+ proportion in steady state, , to a linear regression problem,
Here, we use that the absolute and relative abundance of the CD4+FOXP3+Treg population are coupled by (see Materials and methods). We assume that the steady-state CD4+ T-cell concentration is constant, , across all microbial compositions. We justify this because we are dealing with genetically similar mice and a set of closely related Clostridia. This assumption is however not justifiable when comparing non-colonized and colonized germ-free mice (Faith et al., 2014).
Assigning to the measured FACS-derived CD4+FOXP3+ Treg proportions after 35 days and to the corresponding microbial profiles, we infer each strain's contribution to the CD4+FOXP3+ Treg pool (Figure 2B) by solving Equation (2) with an -penalized least-square regression with one shrinkage parameter, which is determined in a leave-one-sample-out cross-validation (Stein et al., 2013). The resulting normalized root-mean square deviation on left-out samples was found to be 12%.
Derivation of the Treg Induction Score (TrIS) and selection of Treg-inducing consortia
After deriving a model to predict our candidate strains dynamics in germ-free conditions and having resolved each strain’s contribution to Treg expansion, we aim to use this information to computationally select for consortia that maximize Treg induction while being ecologically robust (Bucci et al., 2016; Stein et al., 2013). To specify a measure of ecological robustness as well as immune induction potential for microbial consortia in germ-free mice, we define the Treg Induction Score (TrIS) as the average predicted regulatory T-cell activation of a given consortium of K strains while ignoring contributions from the host,
If the predicted steady state of the microbial consortium that is computed from the -th Markov Chain Monte Carlo (MCMC) parameter estimate (Bucci et al., 2016) is biologically meaningful, i.e. positive, and stable, then denotes the steady-state concentration of strain ; otherwise is set to 0. Hence, the value of the TrIS is indicative of the expected CD4+FOXP3+ Treg induction (after removing the host contribution) and it is of the same unit as the FACS measurements. We evaluated TrIS for every possible strain combination that would stably colonize the gut in germ-free background. In our computation, of the 212–1 = 4095 possible steady-state strain configurations evaluated in N = 22,500 MCMC parameter estimates, 84% are found to be biologically meaningful and stable. Interestingly, while the average TrIS increases with consortium size, our analysis shows that a subset size of seven already contains bacterial combinations maximizing induction (Figure 4A). Furthermore, in addition to the strong correlation between TrIS and the predicted total bacterial abundance in the consortium, we observed that high-induction consortia display an especially large enrichment in the abundance of Strain 27 (Figure 4B, Figure 4—figure supplement 1). Because short-chain fatty acids have been previously associated with colonic Treg induction (Arpaia et al., 2013) and increase in density upon supplementation with these strains (Atarashi et al., 2013), we decided to test if modeling-predicted high Treg-inducing consortia were also enriched in SCFAs. We therefore compared the top 5-inducing microbial consortia of size seven against their same-size counterpart bottom 5 (Figure 4C). We predicted the SCFA concentration for each of the predicted compositions by summing the scaled metabolic outputs of each strain measured in mono-colonization experiments (Atarashi et al., 2013; Narushima et al., 2014) and normalized by the strain’s model-predicted mono-colonization density. We performed a Welch two-samples t-test for each of the predicted SCFAs concentrations and found significant enrichment for all estimated SCFAs (p<0.05, one tailed) in the high-TrIS consortia compared to the low ones (Figure 4D).
Experimental validation of mathematical model predictions
We decided to experimentally test our approach's ability to correctly predict consortium ranking with respect to Treg-induction. Due to regulatory constraints on the used probiotic strains – limiting us to a maximum of four strains at a time in follow-up experiments – we selected five 4-strain combinations with a variety of predicted immune effects. We measured CD4+FOXP3+ Treg induction for five microbial consortia of size four and the germ-free control. We chose the two highest TrIS consortia (H1, H2 with rank 1 and 2, respectively), the lowest one (L with rank 495) and two TrIS-intermediate consortia of interest (M1, M2 with rank 129 and 452, respectively). Strains contained in each of the five consortia are detailed in Table 1. We correlated the TrIS score with the mean observed CD4+FOXP3+ Treg percentage and found a significant Pearson correlation with coefficient of 0.97 and p-value<0.01 (Figure 4E). Importantly, when using 16S rRNA sequencing to investigate the resulting colonization profiles for these combinations, we observed that the high TrIS-scoring consortia (H1, H2, M1) all stably colonized while the two low-scoring consortia only displayed a subset of the introduced strains. This result remarkably reflects the nature of our scoring system which, in addition to immune activation potential, also incorporates colonization success (Equation 3).
Because the SCFA enrichment analysis from in vivo measurements of individual strains (Figure 4C) predicted acetate to be significantly increased in the five high versus low Treg-inducing consortia of size seven, we decided to compare the measured acetic acid concentrations (Figure 4—figure supplement 2A) to predictions of acetate in the two high (H1 and H2) and low (L) Treg-inducing 4-strain consortia (Figure 4—figure supplement 2B). ANOVA and subsequent post-hoc Tukey test showed significance in the measured enrichment of H1 compared to L (adjusted p-value<0.05) and of H1 compared to H2 (adjusted p-value<0.05) (Figure 4—figure supplement 2A). However, no statistical difference was observed between measurements in H2 and L (adjusted p-value<0.05). Remarkably, the model-predicted germ-free normalized acetate enrichment and the observed acetic acid concentration (normalized by the mean in germ-free conditions) are well correlated (Figure 4—figure supplement 2B).
Discussion
Manipulation of the intestinal microbiota with defined bacterial consortia for the treatment of disease is a promising route for future therapeutics (Hansen and Sartor, 2015). However, choosing bacterial combinations from the vast combinatorial space of microbes that effectively colonize a host (possibly with a dysbiotic microbiota) and at the same time maximize a desired host phenotype requires an enormous number of expensive and time-consuming experimental trials (Faith et al., 2014). While data mining and statistical approaches could aid in this process, the majority of available microbiome analysis methods is still based on correlations (Gerber, 2014; Morgan et al., 2015) and inept at predicting unseen phenotypes. Identification of causal associations between microbes and host phenotype has been recently achieved by using an experimentally based microbe–phenotype triangulation (Surana and Kasper, 2017). However, this approach remains impractical when the goal is to explore and rank all possible microbiome consortium combinations with respect to a host phenotype of interest. In this study, we leveraged our previous computational methods to forecast temporal microbiome dynamics and make predictions on stability in the context of infectious and inflammatory diseases including C. difficile colonization and inflammatory bowel disease (Bucci et al., 2016; Buffie et al., 2015; Stein et al., 2013).
Specifically, we have presented a novel modeling-based method that combines dynamical predictions from data-driven models of the microbiome and the interactions between microbes and the immune response (such as CD4+FOXP3+ Treg) and thereby enables the rational design of immune-modulating bacterial consortia. Limiting our scope to long-term steady-state dynamics, we were able to derive a microbiome–Treg mathematical model which is constrained by experimental observations from multimodal data. In the presented work, the used data modalities are qPCR and 16S rRNA sequencing data to estimate microbial abundances and FACS to assess CD4+FOXP3+ Treg induction, respectively. However, the proposed framework is naturally extensible to other host data, for example data from metabolite profiling, immune readouts (e.g. CD8 T-cell activation, Th1/Th17 cell depletion) or host transcriptome profiling (Atarashi et al., 2017; Morgan et al., 2015). To evaluate candidate consortia that could be relevant to the treatment of auto-inflammatory diseases, we introduced the Treg Induction Score, TrIS, as a novel metric that accounts for both ecological stability and efficacy in immune modulation. This metric allowed us to rationally identify combinations that would stably colonize and at the same time produce substantial Treg induction in germ-free mice. Remarkably, in validation experiments of five distinct modeling-guided consortia with predicted diverse induction potential, we proved the ability of our approach to successfully select microbial combinations with a desired therapeutic activity. To our knowledge, this is to date, the first study in which observation-constrained in silico modeling of microbiome and host phenotype has successfully guided the rational design of drugs of defined bacterial consortia.
Our work relies on the gLV model assumption for both microbiome and CD4+ regulatory T-cell dynamics which, despite being very versatile, has some limitations including the lack of third or higher order interactions or saturation effects (Wangersky, 1978). Moreover, with the data available to us, we needed to assume that the overall T-cell density at steady state is constant across mice and different microbiome compositions. While this assumption was reasonable for the data we analyzed (see Results), the addition of future measurements on total T-cell abundance will likely improve the prediction accuracy of our model as well as provide important insight into the variability of T-cell population size in response to microbial immune induction.
We performed our data and modeling analysis in germ-free mice. While previous work by some of us showed that the amount of Treg induction is independent of the germ-free background (IQI, Balb/c or C57BL/6) (Atarashi et al., 2015), it is noteworthy that our model, which was trained on IQI mice data, robustly predicted both Treg induction and acetate enrichment of C57BL/6 colonized mice as used in the validation experiments.
Our predictions suggest some degree of consistency in terms of functional outcome (e.g. enrichment in acetate) of high-scoring consortia relative to low-scoring ones in germ-free mice. However, before translating these findings into therapeutic development, future studies will need to be performed in not germ-free settings (e.g. SPF mice, humanized mice) in order to account for the effects of an already-established flora. Individual-specific microbiome features may constrain the colonization potential of the selected strains due to specific ecological network effects (Smits et al., 2016), which suggests the need for a careful characterization of the ecological interactions between the proposed probiotic product and a specific recipient community (e.g. a single ulcerative colitis patient (Atarashi et al., 2013)).
Treatment of auto-immune diseases overall is not necessarily achieved by the exclusive optimization of one objective function (e.g. for ulcerative colitis, the maximization of Treg activation), but may need the simultaneous manipulation of a multi-process host immune spectrum, which could include the concomitant reduction of pro-inflammatory phenotypes (Atarashi et al., 2015). Given new data availability and careful experimental design, we believe that the modeling framework proposed in this study could overcome these problems by introducing constraints into the scoring metric that account for pre-colonized mouse model features. To the limit, in the presence of a large training data set that would include sufficient information on individual patient variation, our workflow will give us the unprecedented potential of testing microbiota manipulation on a personalized level in silico.
Mathematical modeling-based methods have the potential to greatly accelerate the development of treatment of human disease (Michor and Beal, 2015). In this work, we develop and demonstrate in a first-of-a-kind experimental validation the usefulness of a mathematical microbiome–immune system model. It can be considered a stepping-stone to the accelerated prototyping and rational design of microbiome therapies.
Materials and methods
Derivation of the microbiome–Treg induction mathematical model
Request a detailed protocolWe assume the following dynamics for the CD4+FOXP3+ regulatory T-cell population:
Here, denotes the basal growth rate and the self-interaction term of the CD4+FOXP3+ Treg population, while the interaction parameters and characterize the effect of the CD4+FOXP3− T-cells on the CD4+FOXP3+ Treg population and of the CD4+FOXP3+ Treg on the CD4+FOXP3− T-cell population, respectively (d’Onofrio, 2005). Moreover, denotes the effect of strain on the CD4+FOXP3+ Treg population. The non-trivial steady-state solution (i.e., the algebraic solution of the right-hand side of Equation (1) set to 0 with ) is found as,
Using the steady-state concentrations of the CD4+FOXP3+ Treg and CD4+FOXP3− populations, , are derived from the FACS-based relative abundances by, . Finally, the linear relationship between the relative abundances, , and the strain densities, , is found as,
assuming constant concentration of CD4+ T-cells, , across all possible microbiome compositions. The unknown parameters and are estimated in an -penalized least-square regression (so-called ridge regression) with a positive shrinkage parameter , which is determined in a leave-one-sample-out cross-validation as .
Collection of experimental data for training the microbiome–immune system model
Strain abundance profiling
Request a detailed protocolIn our previous work (Bucci et al., 2016), we have inferred a mathematical model describing the dynamics of a 13-strain subset from the original 17-strain human-derived Clostridia consortium from Atarashi et al. (2013) in germ-free mice. Using newly generated data from the same experimental setup, we are now able to assess its predictive quality for three distinct 11-strain subsets. The three 11-strain compositions were selected based on their capability to maintain stability in the simulations when either one of two high (Strain 15 and Strain 4, here referred to as cases I and II, respectively) or one low (Strain 29; III) keystone strain was removed (Bucci et al., 2016). For the experimental validation, germ-free IQI mice were purchased from Sankyo Laboratories (Japan), randomized and maintained in germ-free vinyl isolators in the animal facility of RIKEN. Twelve Treg-inducing Clostridia strains were selected from the previously reported VE202 consortium consisting of 17 Treg-inducing strains (4, 7, 9, 13–16, 21, 26–29) and were individually cultured in modified Eggerth Gagnon broth under strictly anaerobic conditions (80% N2, 10% H2, 10% CO2) at 37°C in an anaerobic chamber (Coy Laboratory Products, Grass Lake, MI) to confluence. The cultured bacterial strains were then mixed and the three mixtures of 11 strains (described above) were orally inoculated into five IQI germ-free adult mice each. One mouse for condition II died and was therefore discarded from the study. After an initial 9-day interval of acclimation, we collected fecal pellets at 2–4 days interval until day 35, time at which mice were euthanized and analyzed for CD4+FOXP3 induction as in Atarashi et al. (2013). Colonization levels for each strain were assessed by amplifying strain-specific regions with qPCR as described in Bucci et al. (2016). Bacterial genomic DNA was extracted from 1 to 2 fecal pellets using QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany). The amount of DNA was quantified using a Qubit dsDNA HS assay kit and Qubit fluorometer (Invitrogen, Carlsbad, CA). DNA was then subjected to qPCR using Thunderbird SYBR qPCR Mix (TOYOBO, Osaka, Japan) and a LightCycler 480 (Roche) with primers specific to 16S ribosomal RNA (rRNA) genes of the 12 Clostridia strains as in Bucci et al. (2016). Quantification of each strain in each sample was accomplished using standard curves of known concentrations of DNAs purified from each strain individually cultured in vitro. Strain densities in each sample were calculated by dividing the above absolute quantification numbers by the weight of the extracted fecal DNA. The 11-strain experiments were ethically approved by RIKEN, Keio and Azabu Universities under protocol H24-9(14) from RIKEN.
Estimation of CD4+FOXP3+ Treg – Isolation of intestinal lamina propria lymphocytes and flow cytometry
Request a detailed protocolThe colons were collected and opened longitudinally, washed with PBS to remove all luminal contents and shaken in Hanks’ balanced salt solution (HBSS) containing 5 mM EDTA for 20 min at 37°C. After removing epithelial cells, muscle layers and fat tissue using forceps, the lamina propria layers were cut into small pieces and incubated with RPMI1640 containing 4% fetal bovine serum, 0.5 mg/ml collagenase D, 0.5 mg/ml dispase and 40 mg/ml DNase I (all Roche Diagnostics, Risch-Rotkreuz, Switzerland) for 1 hr at 37°C in a shaking water bath. The digested tissues were washed with HBSS containing 5 mM EDTA, resuspended in 5 ml of 40% Percoll (GE Healthcare, Boston, MA) and overlaid on 2.5 ml of 80% Percoll in a 15 ml Falcon tube. Percoll gradient separation was performed by centrifugation at 850 g for 25 min at 25°C. The lamina propria lymphocytes were collected from the interface of the Percoll gradient and suspended in ice-cold PBS. For analysis of Treg, isolated lymphocytes were labeled with the LIVE/DEAD fixable dead cell stain kit (Life Technologies, Carlsbad, CA) to exclude dead cells from the analysis. Then, surface and intracellular staining of CD3, CD4 and FOXP3 was performed using the BV605-labeled anti-CD3 (17A2, Biolegend, San Diego, CA), BV421-labeled anti-CD4 (RM4-5, Biolegend), Alexa700-labeled anti-FOXP3 antibody (FJK-16 s, eBioscience, San Diego, CA), and FOXP3 staining buffer set (eBioscience). The antibody-stained cells were analyzed with LSR Fortessa and data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Measurement of organic acids
Request a detailed protocolOrganic acid concentrations in caecal contents were determined by gas chromatography-mass spectrometry (GC-MS). Caecal contents (10 mg) were disrupted using 3 mm zirconia/silica beads (BioSpec Products) and homogenized in extraction solution containing 100 ml of internal standard (100 mM crotonic acid), 50 ml of HCl and 200 ml of ether. After vigorous shaking using a Shakemaster neo (Bio Medical Science) at 1500 rpm for 10 min, homogenates were centrifuged at 1000 g for 10 min and then the top ether layer was collected and transferred into new glass vials. Aliquots (80 ml) of the ether extracts were mixed with 16 ml of N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide (MTBSTFA). The vials were sealed tightly by screwing and heated at 80°C for 20 min in a water bath, and left at room temperature for 48 hr for derivatization. The samples were then run through a 6890N Network GC System (Agilent Technologies) equipped with HP-5MS column (0.25 mm 330 m 30.25 mm) and 5973 Network Mass Selective Detector (Agilent Technologies, Santa Clara, CA). Pure helium (99.9999%) was used as a carrier gas and delivered at a flow rate of 1.2 ml/min. The head pressure was set at 10 psi with split 10:1. The inlet and transfer line temperatures were 250 µC and 260 µC, respectively. The following temperature program was used: 60 µC (3 min), 60–120°C (5°C/min), 120–300°C (20°C/min). One microliter quantity of each sample was injected with a run time of 30 min. Organic acid concentrations were quantified by comparing their peak areas with the standards.
Numerical simulations of the three 11-strain subsets used for microbiome–Treg model training
Request a detailed protocolWe used 22,500 sets of Markov Chain Monte Carlo generalized Lotka–Volterra parameter sets determined by applying the Bayesian Variable Selection algorithm within MDSINE to the data of Bucci et al. (2016), and the first time point of measured microbial profiles for each of the 14 validation mice as initial condition, to simulate the gLV system of differential equations corresponding to each mouse microbiome (Figure 3A). Prediction accuracy was evaluated by calculating the Spearman correlation coefficient between observed and predicted data (Figure 3B).
Simulation of mono-colonization abundances
Request a detailed protocolAs the experimental data from Atarashi et al. (2013) only provided CD4+FOXP3+ T-cell levels for the mono-strain colonization experiments at 35 days after inoculation but no measurement of the long-term microbial concentrations in the gut, we used instead the corresponding estimated mono-strain colonization densities obtained from the gLV model (section above and Figure 2A). In general, the long-term behavior of the gLV system is determined by its steady states which are uniquely defined by the inferred model parameters (Stein et al., 2013). We computed the parameter median in each single model variable from the 22,500 MCMC parameter sets. These median parameters were then used to deduce the steady-state densities, which were together with the measured CD4+FOXP3 proportions included into the training of the microbiome–Treg model.
Collection of CD4+FOXP3+ Treg data from 4-strain experiments for validation of the modeling-based predictions
Request a detailed protocolBacterial strains 4, 7, 9, 14, 15, 16, 27, 28, 29 were grown anaerobically in PYG broth (Peptone, Yeast and Glucose broth from Anaerobe Systems, Cat no: AS-822) until they reached stationary phase (48 hr for strains 27 and 29, 24 hr for the remaining strains). Each 200 µl-mouse dose of a 4-strain LBP contained 50 µl of 20 times concentrated stationary phase culture. Germ-free C57BL/6 mice aged 6–8 weeks were randomized and gavaged with a total dose of 5·107–2·108 bacteria in a 200 µl, and maintained under gnotobiotic conditions for four weeks. Use of a C57BL/6 background for these experiments was motivated by availability of animals at the facility where we performed the validation and justified by the fact that previous work from us has shown that Treg induction by our Clostridia strains does not differ between Balb/c, IQI, and C57BL/6 mice (Atarashi et al., 2015). Mice were then sacrificed, colons harvested, and lamina propria leukocytes isolated and stained for CD3+CD4+FOXP3+ Treg as described above. Eight mice each were used for consortia High 1 (H1-strains: 7, 27, 28, 29) and High 2 (H2-strains: 4, 7, 27, 29). Five mice each were used for the intermediate high (M1-strains: 4, 7, 14, 28), and intermediate low consortia (M2-strains: 9, 16, 27, 29). Three mice were used for Low 1 (L1-strains: 14, 15, 16, 29). Colonization profiling was determined through 16S rRNA sequencing (as above) and verified by blasting representative sequences to a 16S VE202 fasta database. The 4-strain validation experiments were performed in the Massachusetts Host Microbiome Center under IACUC protocol 2016N000141.
Data availability
-
Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial CellsPublicly available at the DDBJ Center (accession no. DRA003884).
References
-
Induced CD4+Foxp3+ regulatory T cells in immune toleranceAnnual Review of Immunology 30:733–758.https://doi.org/10.1146/annurev-immunol-020711-075043
-
Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic miceScience Translational Medicine 6:ra11.https://doi.org/10.1126/scitranslmed.3008051
-
The treatment-naive microbiome in new-onset Crohn's diseaseCell Host & Microbe 15:382–392.https://doi.org/10.1016/j.chom.2014.02.005
-
Therapeutic manipulation of the microbiome in IBD: Current results and future approachesCurrent Treatment Options in Gastroenterology 13:105–120.https://doi.org/10.1007/s11938-014-0042-7
-
The microbiome in infectious disease and inflammationAnnual Review of Immunology 30:759–795.https://doi.org/10.1146/annurev-immunol-020711-074937
-
Immune recognition and response to the intestinal microbiome in type 1 diabetesJournal of Autoimmunity 71:10–18.https://doi.org/10.1016/j.jaut.2016.02.004
-
Regulatory T cells and Foxp3Immunological Reviews 241:260–268.https://doi.org/10.1111/j.1600-065X.2011.01018.x
-
Development and maintenance of intestinal regulatory T cellsNature Reviews Immunology 16:295–309.https://doi.org/10.1038/nri.2016.36
-
Duodenal infusion of donor feces for recurrent Clostridium difficileNew England Journal of Medicine 368:407–415.https://doi.org/10.1056/NEJMoa1205037
-
New insights into therapeutic strategies for gut microbiota modulation in inflammatory diseasesClinical & Translational Immunology 5:e87.https://doi.org/10.1038/cti.2016.38
-
Lotka-volterra population modelsAnnual Review of Ecology and Systematics 9:189–218.https://doi.org/10.1146/annurev.es.09.110178.001201
-
Fecal microbiota transplant: benefits and risksOpen Forum Infectious Diseases 2:ofv005.https://doi.org/10.1093/ofid/ofv005
Article and author information
Author details
Funding
National Institutes of Health (P41 GM103504)
- Richard R Stein
- Chris Sander
Defense Advanced Research Projects Agency (BRICS award HR0011-15-C-0094)
- Georg K Gerber
Human Frontier Science Program (RGP00055/2015)
- Chris Sander
Takeda Science Foundation
- Kenya Honda
National Institute of General Medical Sciences (5R01 GM106303)
- Chris Sander
Japan Agency for Medical Research and Development
- Kenya Honda
National Institute of Allergy and Infectious Diseases (R15-AI112985-01A1)
- Vanni Bucci
National Science Foundation (1458347)
- Vanni Bucci
Core Research for Evolutional Science and Technology
- Kenya Honda
Brigham and Women's Hospital (Precision Medicine Initiative)
- Georg K Gerber
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Ethics
Animal experimentation: 11-strain time-series mouse experiments were performed under ethical approval by RIKEN, Keio and Azabu Universities under protocol H24-9(14) (RIKEN). 4-strain validation mouse work was performed at Brigham and Women's Hospital in Boston, MA in the Massachusetts Host Microbiome Center under IACUC protocol 2016N000141.
Copyright
© 2018, Stein et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 7,991
- views
-
- 1,371
- downloads
-
- 71
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Computational and Systems Biology
In eukaryotes, protein kinase signaling is regulated by a diverse array of post-translational modifications, including phosphorylation of Ser/Thr residues and oxidation of cysteine (Cys) residues. While regulation by activation segment phosphorylation of Ser/Thr residues is well understood, relatively little is known about how oxidation of cysteine residues modulate catalysis. In this study, we investigate redox regulation of the AMPK-related brain-selective kinases (BRSK) 1 and 2, and detail how broad catalytic activity is directly regulated through reversible oxidation and reduction of evolutionarily conserved Cys residues within the catalytic domain. We show that redox-dependent control of BRSKs is a dynamic and multilayered process involving oxidative modifications of several Cys residues, including the formation of intramolecular disulfide bonds involving a pair of Cys residues near the catalytic HRD motif and a highly conserved T-loop Cys with a BRSK-specific Cys within an unusual CPE motif at the end of the activation segment. Consistently, mutation of the CPE-Cys increases catalytic activity in vitro and drives phosphorylation of the BRSK substrate Tau in cells. Molecular modeling and molecular dynamics simulations indicate that oxidation of the CPE-Cys destabilizes a conserved salt bridge network critical for allosteric activation. The occurrence of spatially proximal Cys amino acids in diverse Ser/Thr protein kinase families suggests that disulfide-mediated control of catalytic activity may be a prevalent mechanism for regulation within the broader AMPK family.
-
- Computational and Systems Biology
- Genetics and Genomics
Obesity is a major risk factor for type 2 diabetes, dyslipidemia, cardiovascular disease, and hypertension. Intriguingly, there is a subset of metabolically healthy obese (MHO) individuals who are seemingly able to maintain a healthy metabolic profile free of metabolic syndrome. The molecular underpinnings of MHO, however, are not well understood. Here, we report that CTRP10/C1QL2-deficient mice represent a unique female model of MHO. CTRP10 modulates weight gain in a striking and sexually dimorphic manner. Female, but not male, mice lacking CTRP10 develop obesity with age on a low-fat diet while maintaining an otherwise healthy metabolic profile. When fed an obesogenic diet, female Ctrp10 knockout (KO) mice show rapid weight gain. Despite pronounced obesity, Ctrp10 KO female mice do not develop steatosis, dyslipidemia, glucose intolerance, insulin resistance, oxidative stress, or low-grade inflammation. Obesity is largely uncoupled from metabolic dysregulation in female KO mice. Multi-tissue transcriptomic analyses highlighted gene expression changes and pathways associated with insulin-sensitive obesity. Transcriptional correlation of the differentially expressed gene (DEG) orthologs in humans also shows sex differences in gene connectivity within and across metabolic tissues, underscoring the conserved sex-dependent function of CTRP10. Collectively, our findings suggest that CTRP10 negatively regulates body weight in females, and that loss of CTRP10 results in benign obesity with largely preserved insulin sensitivity and metabolic health. This female MHO mouse model is valuable for understanding sex-biased mechanisms that uncouple obesity from metabolic dysfunction.






