Parkinson’s Disease: Linking mitochondria to the immune response
Parkinson’s disease is a motor disorder caused by the loss of a specific sub-set of neurons located in the midbrain and the accumulation of a protein called α-synuclein. The underlying mechanisms that lead to the death of the midbrain neurons are still not well understood. However, many individuals with Parkinson’s disease have increased levels of inflammation in the brain and in peripheral organs, such as the gut. This inflammation is now viewed as a potential contributor to Parkinson’s disease, and not just a result of it.
Mutations in the gene coding for the kinase LRRK2 are the most common genetic cause of inherited Parkinson’s disease (Singleton et al., 2013). Single nucleotide polymorphisms in this gene are also associated with increased susceptibility to inflammatory diseases such as leprosy – which, like tuberculosis, is caused by a mycobacterium – and irritable bowel disease (Fava et al., 2016; Witoelar et al., 2017; Villumsen et al., 2019). Mounting evidence suggests that mutations in LRRK2 contribute to immune alterations in both peripheral organs and the brain (Wallings and Tansey, 2019), but the mechanisms through which LRRK2 regulates these immune responses are poorly understood.
Aside from its link to inflammation, LRRK2 has also been implicated in the activity of mitochondria, which are key subcellular organelles linked to Parkinson’s disease (Singh et al., 2019; Park et al., 2018). For example, neural stem cells derived from the skin of individuals carrying a mutation associated with Parkinson’s disease in the gene for LRRK2 exhibit increased mitochondrial DNA damage as well as increased oxidative damage (Howlett et al., 2017; Sanders et al., 2014). Despite this, the contribution of LRRK2 to mitochondrial health in cells of the peripheral immune system has been understudied. Now, in eLife, Robert Watson and colleagues from Texas A&M University – including Chi Weindel and Samantha Bell as joint first authors – report that LRRK2’s ability to influence inflammatory responses in peripheral immune cells is directly linked to its role in maintaining mitochondrial homeostasis (Weindel et al., 2020).
To investigate the role of LRRK2 in peripheral immune responses, Weindel et al. isolated macrophages from the bone marrow of mice lacking the gene that codes for the mouse homolog of LRRK2 (Lrrk2 knockout macrophages). Compared to macrophages with a single copy of this gene (Lrrk2 HETs), the knockout macrophages upregulated genes normally stimulated by interferons (signaling proteins that recruit immune cells to trigger an immune response). Weindel et al. also found that these macrophages were unable to upregulate interferon response genes when they were infected with mycobacteria, likely because these genes were already chronically activated.
Moreover, when mice lacking Lrrk2 were infected with Mycobacterium tuberculosis, which causes tuberculosis, their lungs exhibited exacerbated local inflammation caused by the infection. However, no differences in the outcome of the infection were observed in these mice relative to the Lrrk2 HETs. This contradicts previous reports suggesting that mice lacking Lrrk2 can stop M. tuberculosis replication more effectively than wild-type mice, correlating with increased inflammation in the lungs (Härtlova et al., 2018). One possible reason for this inconsistency is that Weindel et al. used Lrrk2 heterozygous controls as opposed to wild-type controls. Furthermore, different M. tuberculosis strains were used in the two studies, and there were also differences in the number of bacteria that mice were exposed to during infection.
Since LRRK2 is also involved in mitochondrial activity, Weindel et al. next looked at the mitochondria in Lrrk2 knockout macrophages. Mitochondria are dynamic organelles that can divide (fragment) or fuse depending on the state of the cell. When a cell is stressed, mitochondria fuse together to exchange damaged DNA and keep aerobic respiration going. Weindel et al. observed that in macrophages lacking Lrrk2, mitochondria did not fuse, making the macrophages more susceptible to stress, and leading to mitochondrial DNA leaking into the cytosol. This was due, in part, to increased activation of DRP1, a protein that helps mitochondria fragment. Inhibiting DRP1 successfully rescued these abnormal mitochondria in Lrrk2 knockout macrophages. Weindel et al. also reported reduced antioxidant levels, a concomitant accumulation of reactive oxygen species, and increased mitochondrial stress in macrophages lacking Lrrk2. Treating these macrophages with antioxidants alleviated mitochondrial stress (Figure 1).
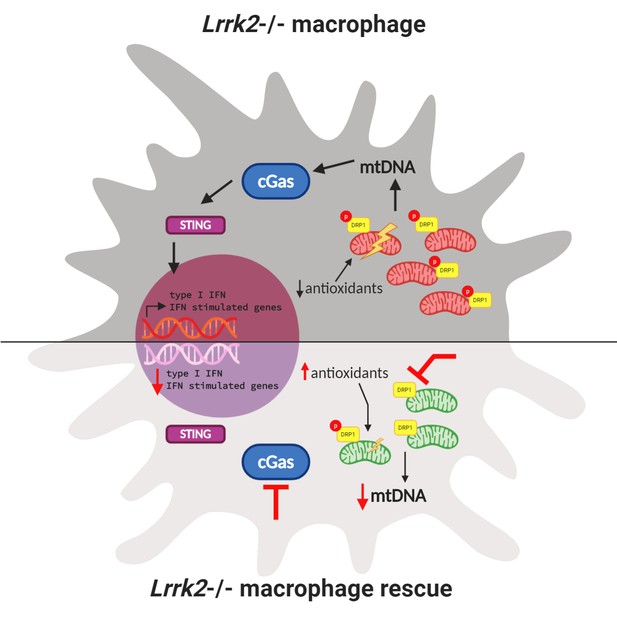
Interactions between mitochondrial homeostasis and immune response in a Lrrk2 knockout macrophage.
(Top) Loss of Lrrk2 in mouse macrophages increases DRP1 phosphorylation. This leads to an increase in the level of mitochondrial DNA (mtDNA; red bean-shaped structures) in the cytosol, which activates the cGAS/STING pathway. This, in turn, triggers the phosphorylation of transcription factors that activate the expression of interferon (IFN) response genes in the nucleus, which leads to a lowered response to interferon from these cells. In this situation, antioxidant levels in the cell are low and mitochondrial stress increases. (Bottom) Three different treatments can partially or totally rescue inflammatory deficits in Lrrk2 knockout macrophages. First, treating these macrophages with antioxidants alleviates mitochondrial stress and rescues the normal response to interferon. Second, inhibiting DRP1 phosphorylation decreases fission, lowering the level of mitochondrial DNA (green bean-shaped structures) in the cytosol. This prevents the activation of the cGAS/STING pathway, and consequently, the abnormal activation of interferon response genes. Third (and confirming this finding), removing the cGas gene also mitigates increased interferon stimulated gene expression.
The increased mitochondrial DNA in the cytosol of Lrrk2 knockout macrophages led Weindel et al. to hypothesize that the inability to regulate mitochondrial homeostasis could be contributing to chronic activation of interferon response genes. The cGAS–STING pathway is a part of the innate immune system that triggers the expression of inflammatory genes when DNA is detected in the cytosol. Weindel et al. showed that loss of the cGas gene decreased basal levels of type I interferon in Lrrk2 knockout macrophages and corrected the response to immune stimuli (Figure 1).
These findings link the role of LRRK2 in innate immune dysregulation with its critical function in maintaining mitochondrial homeostasis for the first time and have wider implications for the field of Parkinson’s disease. Future research will likely investigate how mutations in the gene for LRRK2 affect the relationship between mitochondria and inflammation in immune cells. The most common of these mutations causes a toxic increase in kinase activity, so kinase inhibitors have been considered for their potential therapeutic effects in Parkinson’s disease. However, given that loss of LRRK2 may lead to higher risks of infection and inflammation in peripheral blood cells, therapeutic windows will have to be carefully monitored to avoid unwanted effects on the immune system.
References
-
A missense LRRK2 variant is a risk factor for excessive inflammatory responses in leprosyPLOS Neglected Tropical Diseases 10:e0004412.https://doi.org/10.1371/journal.pntd.0004412
-
Mitochondrial dysfunction in Parkinson's disease: new mechanistic insights and therapeutic perspectivesCurrent Neurology and Neuroscience Reports 18:21.https://doi.org/10.1007/s11910-018-0829-3
-
LRRK2 and mitochondria: recent advances and current viewsBrain Research 1702:96–104.https://doi.org/10.1016/j.brainres.2018.06.010
-
The genetics of Parkinson's disease: progress and therapeutic implicationsMovement Disorders 28:14–23.https://doi.org/10.1002/mds.25249
-
LRRK2 regulation of immune-pathways and inflammatory diseaseBiochemical Society Transactions 47:1581–1595.https://doi.org/10.1042/BST20180463
Article and author information
Author details
Publication history
Copyright
© 2020, Wallings et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,788
- views
-
- 350
- downloads
-
- 7
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Immunology and Inflammation
- Structural Biology and Molecular Biophysics
Antibodies are a major component of adaptive immunity against invading pathogens. Here, we explore possibilities for an analytical approach to characterize the antigen-specific antibody repertoire directly from the secreted proteins in convalescent serum. This approach aims to perform simultaneous antibody sequencing and epitope mapping using a combination of single particle cryo-electron microscopy (cryoEM) and bottom-up proteomics techniques based on mass spectrometry (LC-MS/MS). We evaluate the performance of the deep-learning tool ModelAngelo in determining de novo antibody sequences directly from reconstructed 3D volumes of antibody-antigen complexes. We demonstrate that while map quality is a critical bottleneck, it is possible to sequence antibody variable domains from cryoEM reconstructions with accuracies of up to 80–90%. While the rate of errors exceeds the typical levels of somatic hypermutation, we show that the ModelAngelo-derived sequences can be used to assign the used V-genes. This provides a functional guide to assemble de novo peptides from LC-MS/MS data more accurately and improves the tolerance to a background of polyclonal antibody sequences. Following this proof-of-principle, we discuss the feasibility and future directions of this approach to characterize antigen-specific antibody repertoires.
-
- Immunology and Inflammation
The prevalence of metabolic dysfunction-associated steatohepatitis (MASH) is increasing, urging more research into the underlying mechanisms. MicroRNA-26b (Mir26b) might play a role in several MASH-related pathways. Therefore, we aimed to determine the role of Mir26b in MASH and its therapeutic potential using Mir26b mimic-loaded lipid nanoparticles (LNPs). Apoe-/-Mir26b-/-, Apoe-/-Lyz2creMir26bfl/fl mice, and respective controls were fed a Western-type diet to induce MASH. Plasma and liver samples were characterized regarding lipid metabolism, hepatic inflammation, and fibrosis. Additionally, Mir26b mimic-loaded LNPs were injected in Apoe-/-Mir26b-/- mice to rescue the phenotype and key results were validated in human precision-cut liver slices. Finally, kinase profiling was used to elucidate underlying mechanisms. Apoe-/-Mir26b-/- mice showed increased hepatic lipid levels, coinciding with increased expression of scavenger receptor a and platelet glycoprotein 4. Similar effects were found in mice lacking myeloid-specific Mir26b. Additionally, hepatic TNF and IL-6 levels and amount of infiltrated macrophages were increased in Apoe-/-Mir26b-/- mice. Moreover, Tgfb expression was increased by the Mir26b deficiency, leading to more hepatic fibrosis. A murine treatment model with Mir26b mimic-loaded LNPs reduced hepatic lipids, rescuing the observed phenotype. Kinase profiling identified increased inflammatory signaling upon Mir26b deficiency, which was rescued by LNP treatment. Finally, Mir26b mimic-loaded LNPs also reduced inflammation in human precision-cut liver slices. Overall, our study demonstrates that the detrimental effects of Mir26b deficiency in MASH can be rescued by LNP treatment. This novel discovery leads to more insight into MASH development, opening doors to potential new treatment options using LNP technology.






