Senescence: A gradual path to mortality
In 1961, Hayflick and Moorhead discovered that human fibroblast cells cultured in the laboratory could only divide a limited number of times, after which they stopped multiplying but remained metabolically active (Hayflick and Moorhead, 1961). It did not matter how the cells were cultured – whether they were repeatedly transferred, or ‘passaged’, into a fresh environment, or if their growth was interrupted by episodes of freezing – the number of divisions they could make was always finite. This state was termed replicative senescence and was found to occur in a range of cell types.
Further research revealed that senescence is caused by the shortening of caps, or ‘telomeres’, on the end of chromosomes (Bodnar et al., 1998). Every time a cell divides, its telomeres shrink until they reach a critical length that stops the cell from multiplying. Once senescent, cells display unique features such as expressing certain proteins, releasing inflammatory molecules, and loosening tightly packed regions of DNA known as heterochromatin (Hernandez-Segura et al., 2018). New evidence showed that senescence is induced by cell stress as well as successive divisions, and that the number of senescent cells increases as tissues age (van Deursen, 2014).
Despite almost 60 years of research, many questions still remain about senescence; for instance, what happens to cells as they transition in to the senescent state? How does their metabolism change during this shift, and do they take on a new cell identity? Now, in eLife, David Botstein, David Hendrickson and colleagues from Calico Life Sciences – including Michelle Chan as first author – report the results of experiments that exquisitely profile the roadmap cells take on their path to senescence (Chan et al., 2022).
The team used a new experimental design to survey the entire genome and repertoire of RNAs, proteins, and metabolites present in fibroblasts cultured in the laboratory. These patterns were traced over time as the cells grew until they stopped dividing. Chan et al. then used a range of control conditions to pinpoint which changes were specific to replicative senescence. This included repeating the experiment on cells growing at a similar density to senescent cultures, cells that had only been passaged a few times (and therefore unlikely to be senescent), cells that never become senescent, and cells made senescent by radiation-induced stress.
To begin with, Chan et al. measured the level of unique RNAs in single cells to investigate how the genes that fibroblasts expressed changed over time. The data revealed that RNAs known to be expressed in fully senescent cells progressively accumulate throughout the cell cycle. This suggests that senescent cells in vivo may be slowly amassing these features, but not yet expressing the classic biomarkers associated with the end-point of senescence, such as the beta-galactosidase enzyme. This may explain why previous studies found less than 20% of cells in old tissues exhibited these biomarkers, which has led researchers to question the role of senescence in aging (Biran et al., 2017; Idda et al., 2020). Chan et al. also found cells that had experienced replicative senescence (but not senescence induced by radiation) expressed genes related to a subtype of epithelial-to-mesenchymal transition (EMT). This process, which sees epithelial cells lining surfaces of the body lose their identity and become mesenchymal, is common in development and cancer.
Further experiments revealed that cells change how they use and generate energy as they progress towards replicative senescence. Similar metabolic changes have also been observed in models of EMT, further validating the connection between replicative senescence and this transition. Chan et al. discovered that the protein complex YAP1/TEAD1 and its target, the growth factor protein TGFβ2, drove this shift in energy. Applying a drug that stops YAP1 and TEAD1 from assembling (Wang et al., 2016) reduced the expression of signatures associated with mesenchymal cells and senescence.
Finally, Chan et al. identified another signature of replicative stress: the increased expression of Nicotinamide N-methyltransferase (NNMT). This enzyme catalyzes chemical reactions that ultimately prevent certain proteins from condensing DNA. It is possible that by reducing the activity of these proteins, NNMT is able to open up closed regions of the genome, which may explain the reported loss of heterochromatin in senescent cells (Yang and Sen, 2018).
The findings of Chan et al. suggest that cells gradually acquire a number of changes on the path to replicative senescence: they express different genes, rewire their metabolic reactions and take on a new identity similar to mesenchymal cells (Figure 1). Previous studies have shown that removing senescent cells can increase the health- and life-span of mice (Di Micco et al., 2021). Therefore, interventions that target these early changes could help improve the wellbeing of individuals by stopping the cascade of events that lead to replicative senescence.
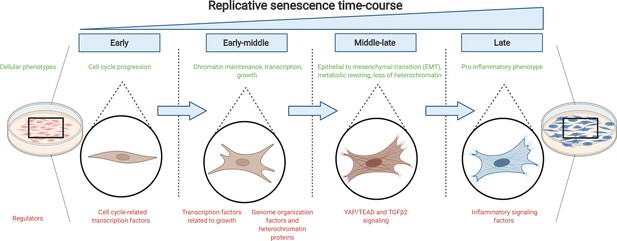
The journey to replicative senescence.
Healthy cells cultured in the laboratory (left) will undergo continuous rounds of division until they reach a state of growth arrest called replicative senescence (right; shown in blue). To begin with (early and early-middle stages), cells express factors associated with cell cycle progression and growth as well as proteins that maintain chromatin stability. As they progress towards replicative senescence (middle-late stage), they undergo an epithelial-to-mesenchymal transition (EMT) and change their cell identity: this transition is driven by the growth factor protein TGFβ2 and the protein complex YAP1/TEAD1. As cells near replicative senescence (late stage), they exhibit a pro-inflammatory phenotype and secrete cytokines, in addition to expressing a beta-galactosidase enzyme which is detected by a reaction that turns cells blue.
Image credit: Figure created using BioRender.
References
-
Cellular senescence in ageing: from mechanisms to therapeutic opportunitiesNature Reviews Molecular Cell Biology 22:75–95.https://doi.org/10.1038/s41580-020-00314-w
-
The serial cultivation of human diploid cell strainsExperimental Cell Research 25:585–621.https://doi.org/10.1016/0014-4827(61)90192-6
-
Hallmarks of cellular senescenceTrends in Cell Biology 28:436–453.https://doi.org/10.1016/j.tcb.2018.02.001
-
Verteporfin inhibits YAP function through up-regulating 14-3-3sigma sequestering YAP in the cytoplasmAmerican Journal of Cancer Research 6:27–37.
Article and author information
Author details
Publication history
Copyright
© 2022, Yang and Sen
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,440
- views
-
- 266
- downloads
-
- 1
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Chromosomes and Gene Expression
- Genetics and Genomics
Among the major classes of RNAs in the cell, tRNAs remain the most difficult to characterize via deep sequencing approaches, as tRNA structure and nucleotide modifications can each interfere with cDNA synthesis by commonly-used reverse transcriptases (RTs). Here, we benchmark a recently-developed RNA cloning protocol, termed Ordered Two-Template Relay (OTTR), to characterize intact tRNAs and tRNA fragments in budding yeast and in mouse tissues. We show that OTTR successfully captures both full-length tRNAs and tRNA fragments in budding yeast and in mouse reproductive tissues without any prior enzymatic treatment, and that tRNA cloning efficiency can be further enhanced via AlkB-mediated demethylation of modified nucleotides. As with other recent tRNA cloning protocols, we find that a subset of nucleotide modifications leave misincorporation signatures in OTTR datasets, enabling their detection without any additional protocol steps. Focusing on tRNA cleavage products, we compare OTTR with several standard small RNA-Seq protocols, finding that OTTR provides the most accurate picture of tRNA fragment levels by comparison to "ground truth" Northern blots. Applying this protocol to mature mouse spermatozoa, our data dramatically alter our understanding of the small RNA cargo of mature mammalian sperm, revealing a far more complex population of tRNA fragments - including both 5′ and 3′ tRNA halves derived from the majority of tRNAs – than previously appreciated. Taken together, our data confirm the superior performance of OTTR to commercial protocols in analysis of tRNA fragments, and force a reappraisal of potential epigenetic functions of the sperm small RNA payload.
-
- Chromosomes and Gene Expression
- Genetics and Genomics
Models of nuclear genome organization often propose a binary division into active versus inactive compartments yet typically overlook nuclear bodies. Here, we integrated analysis of sequencing and image-based data to compare genome organization in four human cell types relative to three different nuclear locales: the nuclear lamina, nuclear speckles, and nucleoli. Although gene expression correlates mostly with nuclear speckle proximity, DNA replication timing correlates with proximity to multiple nuclear locales. Speckle attachment regions emerge as DNA replication initiation zones whose replication timing and gene composition vary with their attachment frequency. Most facultative LADs retain a partially repressed state as iLADs, despite their positioning in the nuclear interior. Knock out of two lamina proteins, Lamin A and LBR, causes a shift of H3K9me3-enriched LADs from lamina to nucleolus, and a reciprocal relocation of H3K27me3-enriched partially repressed iLADs from nucleolus to lamina. Thus, these partially repressed iLADs appear to compete with LADs for nuclear lamina attachment with consequences for replication timing. The nuclear organization in adherent cells is polarized with nuclear bodies and genomic regions segregating both radially and relative to the equatorial plane. Together, our results underscore the importance of considering genome organization relative to nuclear locales for a more complete understanding of the spatial and functional organization of the human genome.






