Unified bursting strategies in ectopic and endogenous even-skipped expression patterns
eLife assessment
This manuscript is an important contribution toward understanding the mechanisms of transcriptional bursting. The evidence is considered solid. Questions regarding the broader advance, details of the analysis, and the models used in the analysis were addressed by the authors.
https://doi.org/10.7554/eLife.88671.3.sa0Important: Findings that have theoretical or practical implications beyond a single subfield
- Landmark
- Fundamental
- Important
- Valuable
- Useful
Solid: Methods, data and analyses broadly support the claims with only minor weaknesses
- Exceptional
- Compelling
- Convincing
- Solid
- Incomplete
- Inadequate
During the peer-review process the editor and reviewers write an eLife Assessment that summarises the significance of the findings reported in the article (on a scale ranging from landmark to useful) and the strength of the evidence (on a scale ranging from exceptional to inadequate). Learn more about eLife Assessments
Abstract
Transcription often occurs in bursts as gene promoters switch stochastically between active and inactive states. Enhancers can dictate transcriptional activity in animal development through the modulation of burst frequency, duration, or amplitude. Previous studies observed that different enhancers can achieve a wide range of transcriptional outputs through the same strategies of bursting control. For example, in Berrocal et al., 2020, we showed that despite responding to different transcription factors, all even-skipped enhancers increase transcription by upregulating burst frequency and amplitude while burst duration remains largely constant. These shared bursting strategies suggest that a unified molecular mechanism constraints how enhancers modulate transcriptional output. Alternatively, different enhancers could have converged on the same bursting control strategy because of natural selection favoring one of these particular strategies. To distinguish between these two scenarios, we compared transcriptional bursting between endogenous and ectopic gene expression patterns. Because enhancers act under different regulatory inputs in ectopic patterns, dissimilar bursting control strategies between endogenous and ectopic patterns would suggest that enhancers adapted their bursting strategies to their trans-regulatory environment. Here, we generated ectopic even-skipped transcription patterns in fruit fly embryos and discovered that bursting strategies remain consistent in endogenous and ectopic even-skipped expression. These results provide evidence for a unified molecular mechanism shaping even-skipped bursting strategies and serve as a starting point to uncover the realm of strategies employed by other enhancers.
Introduction
In animal development, enhancers, cis-regulatory elements that can act at a distance to modulate the transcription of genes (Banerji et al., 1981; Banerji et al., 1983; Gillies et al., 1983) orchestrate the formation of gene expression patterns that dictate animal body plans (Davidson, 2010; Roberta, 2015; Lewis, 1978). At the single-cell level, transcription of most genes has been shown to occur in stochastic pulses, or bursts, of mRNA synthesis (Dar et al., 2012; Golding et al., 2005; McKnight and Miller, 1979; Raj et al., 2006; Senecal et al., 2014; Skupsky et al., 2010; Zenklusen et al., 2008), and patterned developmental genes are no exception (Berrocal et al., 2020; Bothma et al., 2014; Fukaya et al., 2016; Lammers et al., 2020; Zoller et al., 2018). Enhancers typically feature binding sites for several transcription factors proteins. Through these binding sites, enhancers can read out transcription factor concentration and modulate transcriptional bursting dynamics of the genes they regulate (Bothma et al., 2014; Bothma et al., 2015; Chen et al., 2018; Fukaya et al., 2016; Small et al., 1992; Yuh et al., 1994).
Transcriptional bursting can be described by the two-state model of promoter activity (Lionnet and Singer, 2012; Peccoud and Ycart, 1995; Sanchez et al., 2013) that depicts bursts as the result of a gene promoter that switches stochastically between an inactive state, OFF, and an active state, ON, at a rate kon. When the promoter is in its ON state, it loads RNA Pol II molecules onto the gene at a rate r until, eventually, the promoter transitions back to the OFF state at a rate koff and mRNA synthesis stops (Figure 1A and B). In this model, there are multiple distinct ways that enhancers could modulate the rate of mRNA production by tuning transcriptional parameters. For instance, enhancers could upregulate transcription through an increase in burst frequency (kon, also defined as a decrease in the interval between bursts or kon–1), burst duration (koff–1) or burst amplitude (r), or any combination thereof. Recently, quantitative studies have revealed striking similarities in how disparate enhancers modulate these burst parameters to control gene expression. For example, using live-imaging and statistical modeling, we previously showed that the five enhancers that form the seven stripes of even-skipped (eve) expression in Drosophila melanogaster, despite each interacting with a different set of transcription factors, employ the same kinetic strategy to control the rate of mRNA synthesis: they modulate burst frequency and amplitude, while leaving burst duration largely unchanged (Berrocal et al., 2020). Similarly, another study employing single-molecule mRNA FISH suggested that the transcriptional control of various D. melanogaster gap genes is characterized by the shared modulation of burst frequency and duration, while burst amplitude remains constant (Zoller et al., 2018). These two examples suggest a surprising degree of unity—but also of diversity—in the way different enhancers interact with promoters to control transcriptional bursting.
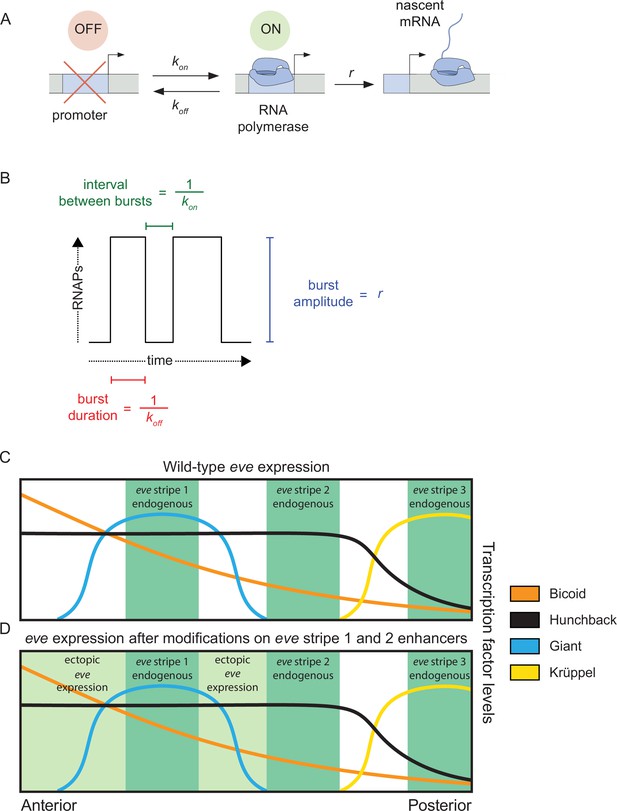
Promoter activity in endogenous and ectopic regions of eve expression.
(A) According to the two-state model of promoter activity a gene promoter switches from the OFF (inactive) state to the ON (active) state at a rate kon. When ON, the promoter loads RNA Pol II molecules and synthesizes mRNA at a rate r. The promoter stochastically switches back to the OFF state at a rate koff. (B) The kon, koff, and r parameters define the average interval between bursts, average burst duration, and average burst amplitude, respectively. (C) eve stripes result from the interplay of various activators and repressors, for instance, wild-type eve stripe 2 is expressed through the interplay of the activators Bicoid and Hunchback with the repressors Giant and Krüppel. The latter define the anterior and posterior boundaries of eve stripe 2, respectively. (D) Here, we coupled the disruption of the eve stripe 1 enhancer with the disruption of the anterior repression of eve stripe 2 exerted by the gap repressor Giant to drive ectopic eve expression anteriorly and compare bursting parameters between endogenous and ectopic expression patterns. (C and D) are based on Levine, 2013 and Peel et al., 2005.
Apparent regulatory unity between various enhancers could be the result of evolutionary adaptation of enhancers to the trans-regulatory inputs that they experience in their endogenous regions of activity. Under this model, we would expect to observe unified bursting strategies at endogenous regions of enhancer activity, while enhancers exposed to non-endogenous regulatory inputs could exhibit different bursting strategies than those observed within their canonical domains of activity. Alternatively, unified strategies of bursting control could result from constraints determined by the biochemistry of the transcriptional processes at enhancers and promoters. In this model, enhancers would control the same set of bursting parameters regardless of the identity and concentration of the input transcription factors at concentrations that enhancers have not encountered during their evolution.
To probe these two models in the context of D. melanogaster development, we used the eve gene as a case study. Our previous work (Berrocal et al., 2020) only examined bursting control strategies in endogenous eve stripes, whose expression dynamics are, in principle, subject to evolutionary selection. To examine expression dynamics in a region presumably devoid of such evolutionary selection, in this study we induced the formation of ectopic eve expression patterns. Specifically, we disrupted two eve enhancers to expand the transcriptional activity of the eve gene onto ectopic regions where enhancers dictate transcriptional bursting in the presence of combinations and concentrations of input transcription factors that D. melanogaster eve enhancers have not encountered in their evolution. We compared bursting parameters in endogenous (Figure 1C) and ectopic regions of eve expression (Figure 1D) and determined that, despite endogenous regions having a higher mean transcriptional output than ectopic regions of eve expression, nuclei in endogenous and ectopic regions modulate their transcriptional output through the same bursting strategies: a concerted increase in promoter kon and r, while koff remains largely unchanged. Our results suggest that eve enhancers have not adapted to yield particular bursting parameters within eve stripes and add further evidence for a unified molecular mechanism behind the modulation of eve transcriptional output. Our work serves as a starting point for uncovering the realm of possible bursting strategies employed by enhancers and opens new research avenues to investigate how these strategies are established at the molecular level.
Results
Mutating eve enhancers to generate ectopic expression patterns
We sought to determine whether eve enhancers regulate transcription by modulating the same set of bursting parameters in endogenous and ectopic eve expression regions. Specifically, we aimed to compare how eve enhancers drive transcriptional bursting in and out of the well-known seven endogenous eve stripes (Frasch and Levine, 1987; Hare et al., 2008).
As our starting point, we took a previously established BAC-based eve-MS2 reporter system (Berrocal et al., 2020) that carries an ~20 kb DNA fragment around the D. melanogaster eve coding region containing the five eve enhancers responsible for regulating the expression of the seven eve stripes, other cis-regulatory elements such as neuronal and muscular regulatory elements (Fujioka et al., 1999; Fujioka et al., 2013) that might influence eve stripe expression in early development (Fujioka et al., 1999; Fujioka et al., 2013), and the late element (LE) that upregulates eve expression in all stripes in response to the EVE protein (Fujioka et al., 1996; Jiang et al., 1991; Figure 2A). We will refer to this construct as eveMS2-BAC (see SI section: DNA constructs and fly lines in Materials and methods). The MS2 reporter system fluorescently labels nascent mRNA molecules resulting in sites of nascent transcription appearing as puncta whose fluorescence is proportional to the number of active RNA Pol II molecules. As a result, the system allows for the visualization of transcriptional bursting at single locus resolution, in real-time, in living embryos (Chubb et al., 2006; Ferguson and Larson, 2013; Garcia et al., 2013; Golding et al., 2005; Golding and Cox, 2004). When inserted into the D. melanogaster genome, eveMS2-BAC expresses in seven stripes that recapitulate the wild-type expression of eve (Figure 2B; Berrocal et al., 2020) as observed by FISH and live-imaging experiments (Lammers et al., 2020; Lim et al., 2018; Luengo Hendriks et al., 2006).
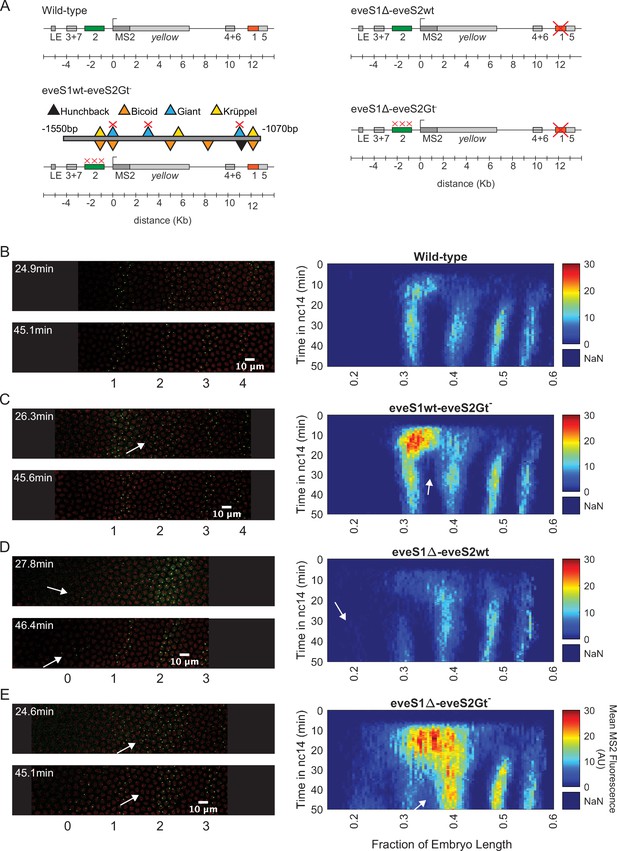
Transcriptional dynamics of eveMS2-BAC variants.
(A) eveMS2 reporter construct variants used in this work. Boxes represent enhancers (e.g. eve stripe 2 enhancer is labeled as 2). LE is the eve late element. eveMS2-BAC is a reporter of wild-type eve expression. The eveS1wt-eveS2Gt- carries a deletion of three Giant binding sites within the eve stripe 2 minimal regulatory element (eveS2-MRE; Small et al., 1992), as indicated by the three red crosses over the eve stripe 2 enhancer, and as shown in the detail of eveS2-MRE; where triangles represent transcription factor-binding sites. The eveS1∆-eveS2wt carries a deletion of the eve stripe 1 enhancer. Finally, eveS1∆-eveS2Gt- combines the Giant binding site deletions from eveS1wt-eveS2Gt- with the eve stripe 1 enhancer deletion of eveS1∆-eveS2wt. (B) Left. Stills from a representative wild-type embryo at ~25 min and ~45 min into nuclear cycle 14 (nc14). Nuclei are labeled in red and transcription sites are labeled in green. Right. Kymograph of eve expression averaged over 5 eveMS2-BAC (wild-type) embryos. Time resolution along the y-axis is 20 seconds. The position of nuclei along the x-axis was calculated from various datasets, based on the inferred position of stripe centers, as described in the SI section: Generation of heatmaps in Figure 2 and Figure 2—figure supplement 1 in Materials and methods. MS2 fluorescence in arbitrary units (AU) along the x-axis was averaged from nuclei located within bins of 0.5% embryo length. (C) Left. eveS1wt-eveS2Gt- embryo at ~25 min and ~45 min into nc14. Right. Average eve-MS2 fluorescence from 6 eveS1wt-eveS2Gt- embryos. At ~25 min, some transcriptionally active nuclei in the inter-stripe region between eve stripe 1 and eve stripe 2 can still be detected (white arrows), while, in wild-type embryos, eve stripe 1 and 2 are completely separated by ~20 min into nc14. (D) Left. eveS1∆-eveS2wt embryo at ~25 min and ~45 min into nc14. Right. Average eve-MS2 fluorescence from 5 eveS1∆-eveS2wt embryos. eve stripe 1 is almost absent at ~25 min, but appears later, probably driven by activity of the eve late element. A dim eve stripe 0 is apparent (white arrows). (E) Left. eveS1∆-eveS2Gt- embryo at ~25 min and ~45 min into nc14. Right. Average eve-MS2 fluorescence from 6 eveS1∆-eveS2Gt- embryos. At ~25 min, there is a strong ectopic expression in the inter-stripe region between eve stripe 1 and eve stripe 2 (white arrow). At ~45 min, this ectopic inter-stripe expression has dimmed (white arrows), while eve stripe 0 becomes apparent.
To establish an ectopic eve expression pattern, we modified the eve reporter locus (Figure 2A; Berrocal et al., 2020). Specifically, we aimed to create an anterior expansion of eve stripe 2 beyond its endogenous expression domain and into ectopic regions where we could study transcriptional bursting under inputs foreign to an eve enhancer, for example higher levels of the activator Bicoid and the repressor Giant (Gt) (Figure 1D). To make this possible, we leveraged the fact that the anterior boundary of eve stripe 2 is established through repression by Giant (Small et al., 1992). Classic work by Small et al. identified a minimal regulatory element of the eve stripe 2 enhancer (eveS2-MRE; Figure 2A) and found that deleting three Giant binding sites within this minimal enhancer produced a strong anterior expansion of eve stripe 2 in the context of a reporter driven by eveS2-MRE (Small et al., 1992).
We generated an eveMS2-BAC variant, where the three binding sites for Giant identified in the eveS2-MRE were disrupted on the complete eve stripe 2 enhancer (eveS1wt-eveS2Gt-; Figure 2A and C). Live imaging experiments on eveS1wt-eveS2Gt- embryos showed only transient ectopic expression at the inter-stripe region between eve stripes 1 and 2. This transient inter-stripe expression lasts until 30–35 min into nc14; while inter-stripe expression between eve stripe 1 and eve stripe 2 disappears after ~20 min in wild-type embryos (compare Figure 2B and C; compare Figure 2—figure supplement 1A and B). These eveS1wt-eveS2Gt- embryos did not produce the robust anterior expansion of eve stripe 2 described for the eveS2-MRE alone (Small et al., 1992). We attribute this muted anterior expansion in eveS1wt-eveS2Gt- embryos (Figure 2C) to the regulatory sequences not present in the original minimal eve stripe 2 reporter construct which might provide a buffering effect to the disruption of the three Giant binding sites (López-Rivera et al., 2020).
In an attempt to expand the anterior ectopic domain of eveS1wt-eveS2Gt-, we sought to free its expression domain from any potential interference from eve stripe 1 expression. To make this possible, we deleted endogenous expression corresponding to the eve stripe 1 enhancer. Specifically, we generated a mutant version of eveMS2-BAC with the eve stripe 1 enhancer deleted (eveS1∆-eveS2wt; Figure 2A and D; Figure 2—figure supplement 1C). Unexpectedly, these embryos still exhibited a dim eve stripe 1 (~30% of embryo length) after ~30 min into nc14, perhaps due to the activity of the eve late element; and a dim additional anterior stripe that we refer to as eve stripe 0 (~20% embryo length) after ~25 min into nc14. In a previous study, Small et al., 1992 identified a ‘head patch’ of gene expression when assaying the regulation of the minimal regulatory element of the eve stripe 2 enhancer. It is tempting to identify our eve stripe 0 with their observed head patch. (Small et al., 1992) speculated that this head patch was the result of sequences in the P-transposon system used for their genomic insertions, which are not present in our experimental design. Thus, the appearance of eve stripe 0 indicates a repressive role of eve stripe 1 enhancer beyond the anterior boundary of eve stripe 1 (Figure 2D), and it may imply that the minimal regulatory element of the eve stripe 2 enhancer can indeed drive expression in this head patch when eve stripe 1 enhancer is not present.
Finally, we coupled the three deletions of Gt-binding sites in the eve stripe 2 enhancer from eveS1wt-eveS2Gt- with the complete deletion of the eve stripe 1 enhancer in eveS1∆-eveS2wt to create eveS1∆-eveS2Gt- (Figure 2A and E; Figure 2—figure supplement 1D). Surprisingly, eveS1∆-eveS2Gt- embryos revealed large ectopic regions of eve expression more complex than the sum of patterns displayed by the independent mutants described above. Beyond a stronger and longer-lasting inter-stripe expression between eve stripe 1 and eve stripe 2 than observed in eveS1wt-eveS2Gt-, eveS1∆-eveS2Gt- embryos exhibited the following notable features: a stronger-than-wild-type eve stripe 2 (located at ~40% of embryo length); the presence of eve stripe 1 (~30% of embryo length) and eve stripe 0 (~20% embryo length); and many eve-active nuclei in normally silent inter-stripe regions between eve stripe 2 and eve stripe 0 (Figure 2E). The fact that the knock-out of eve stripe 1 enhancer coupled with the disruption of Gt-binding sites in eve stripe 2 enhancer renders more ectopic expression on the anterior half of fruit fly embryos than the independent disruptions in eveS1∆-eveS2wt and eveS1wt-eveS2Gt- implies that the repressive activity of the eve stripe 1 enhancer synergizes with the repression exerted by Giant—and potentially with other unidentified transcription factors that bind in the vicinity of Gt-binding sites—on the eve stripe 2 enhancer. The hypothesis that Gt binding sites in eve stripe 2 enhancer may recognize other transcription factors was proposed by Small et al., 1992, who observed that the anterior expansion of eve stripe 2 that results from disrupting Gt-binding sites in eve stripe 2 enhancer is ‘somewhat more severe’ than the expansion observed in Gt- embryos.
Taken together, our results suggest that the eve stripe 1 enhancer plays a repressing role in the anterior half of fruit fly embryos which synergizes with the Giant repressor and likely with other transcriptional regulators bound to Gt binding sites or their vicinity in the eve stripe 2 enhancer. This argues in favor of cross-activity between the eve stripe 1 and 2 enhancers that impacts eve expression in the anterior half of the embryo. eve stripe 1 enhancer might be also playing a role in the regulation of eve stripe 2, as Giant-binding site deletions in the eve stripe 2 enhancer alone do not result in the stronger-than-wild-type eve stripe 2 observed in eveS1∆-eveS2Gt- embryos. In summary, coupling the disruption of Giant-binding sites in the eve stripe 2 enhancer with the deletion of the eve stripe 1 enhancer produces different mutant patterns than the sum of the individual mutants. Finally, regardless of the complex regulatory interactions uncovered by our enhancer mutants, our results indicate that the ectopic gene expression patterns driven by our eveS1∆-eveS2Gt- reporter provide an ideal scaffold for our investigations of the regulation of transcriptional bursting outside of endogenous embryo regions.
Bursting strategies are uniform across endogenous and ectopic eve-active nuclei
We determined the position of nuclei displaying active eve transcription and labeled them as endogenous if they were positioned within the boundaries of wild-type eve stripes (eve stripe 1, eve stripe 2, eve stripe 3, eve stripe 4); or as ectopic if they were located in the inter-stripe region between eve stripe 1 and eve stripe 2 (eve stripe 1–2) or in eve stripe 0 (in the far anterior; Figure 3A) as described in Materials and methods. eve stripe 1 expression in embryos with disrupted eve stripe 1 enhancer was considered endogenous, as we believe that this expression results from activity of the late element. All active nuclei in wild-type embryos were labeled as endogenous. Overall, ectopic regions show lower levels of mean MS2 fluorescence than endogenous regions, as is evident by comparing eve the interstripe 1–2 and eve stripe 0 against eve stripe 1, eve stripe 2, and eve stripe 3 in eveS1∆-eveS2Gt- embryos (Figure 2E, Right). This is perhaps due to the unavailability of optimal concentrations of transcription factors; for example a lack of activators or an excess of repressors with respect to the concentrations found in endogenous regions (Figure 1C and D).
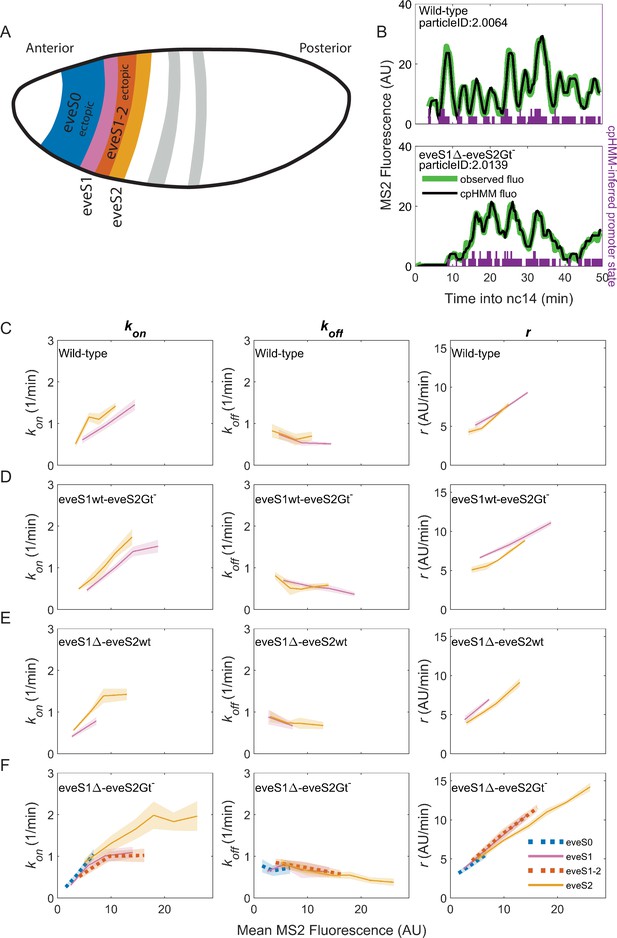
Bursting parameter control is almost identical in endogenous and ectopic gene expression regions.
kon (left panels), koff,(middle panels) and r (right panels) trends across stripes, estimated from nuclei binned by their mean MS2 fluorescence. (A) Position and color code of endogenous and ectopic stripes in the fruit fly embryo. Only eve stripe 0, 1, 1–2, and 2 are shown for clarity. Figure 3—figure supplement 2 includes eve stripe 3, and 4. (B) MS2 fluorescent traces (green) from embryos of different genotypes and cpHMM fit (black). Transcription in Drosophila embryos occurs after DNA replication. Since replicated sister chromatids remain paired, each eve locus contains two promoters, and every one of them can be ON or OFF. Purple bars show cpHMM-inferred promoter state corresponding to the two sister chromatids within a transcription spot (Lammers et al., 2020). Absence of bars represents both sister promoters OFF; shorter bars represent 1 sister promoter ON; longer bars represent 2 sister promoters ON. We aggregated the active state of 1 and 2 sister promoters into a single ON state, which leads to an effective two-state model of promoter activity (see SI section: Inference of Bursting Parameters in Materials and methods). Each point in the plots below was computed from ~40 fluorescent traces. (C) As previously observed in eve-MS2 wild-type embryos (Berrocal et al., 2020), nuclei in all stripes follow the same trends in bursting parameters. kon, the average rate at which the promoter switches from OFF to ON increases with increasing transcriptional initiation as reported by MS2 fluorescence. koff, the average rate at which a promoter switches from ON to OFF remains largely constant, and has a slight decrease in nuclei with the highest MS2 fluorescence values. r, the average rate at which active promoters increase their fluorescence, is higher in brighter nuclei. All stripes from (D) eveS1wt-eveS2Gt- and (E) eveS1∆-eveS2wt share the same bursting strategy. (F) The same trends occur in endogenous (eveS1 and eveS2; solid lines) and ectopic stripes (eveS0 and eveS1-2; dotted lines) of eveS1∆-eveS2Gt- embryos.
To uncover which bursting parameters are modulated to create each endogenous and ectopic stripes and interstripe regions, we need to extract the bursting parameters in each region. We computed bursting parameters for nuclei grouped by stripe and binned by transcriptional output (Figure 3—figure supplement 1) in our four fly lines, with the following rationale. In the bursting model, the average rate of transcription initiation is described by the formula , where indicates the fraction of time the promoter spends in the ON state (Lammers et al., 2020). As enhancers and their inputs (e.g. transcription factors, chromatin state) define bursting parameters (kon, koff, r), nuclei of similar average transcriptional output within the same stripe should be driven by similar inputs acting over the same enhancer. Thus, these nuclei should show similar values of the bursting parameters kon, koff and r that satisfy the equation above. On the other hand, our model predicts that nuclei with different fluorescence must differ in at least one of their bursting parameter values (kon, koff and/or r).
The average MS2 fluorescence is a direct reporter of the average rate of transcriptional initiation. Single-cell MS2 fluorescence measurements reflect the transcriptional dynamics of individual promoters as they undergo transcriptional bursting (Figure 3B). However, the actual promoter states, or bursting parameters, underlying the transcriptional bursting remain ‘hidden’, as RNA Pol II molecules engage in elongation for several minutes (~140 s for the MS2::yellow transcriptional unit in our reporter system) (Berrocal et al., 2020). As a result, MS2 fluorescence is observable even after the promoter switches to the OFF state, convolving the promoter switching dynamics with those of transcriptional elongation. Thus, we can only compute promoter states by inferring them from MS2 fluorescence over time. To infer hidden promoter states, we used a compound-state Hidden Markov Model (cpHMM) developed by Lammers et al., 2020. By inferring the succession of activity states, cpHMM estimates rates of transitioning between the OFF and ON states (kon and koff) and the rate at which ON promoters load active RNA Pol II molecules (r).
Consistent with our previous work (Berrocal et al., 2020), we find that endogenous stripes in eveMS2-BAC wild-type embryos modulate their transcriptional output (mean MS2 fluorescence in wild-type embryos ranges from 2 to 15 AU) by tuning the average kon (from 0.5 to 1.5 OFF to ON promoter transitions per minute) and r (from an average fluorescence increase at a rate of 5 AU per minute to 10 AU per minute). The average koff value remains largely constant (0.5 ON to OFF promoter transitions per minute), with only a minor downregulation at high transcription outputs (Figure 3C). Thus, we confirm that eve-active nuclei in all wild-type stripes achieve higher levels of transcription by upregulating average bursting frequency (kon) and amplitude (r), while average burst duration (koff–1) remains largely the same.
eveS1wt-eveS2Gt- (Figure 3D) and eveS1∆-eveS2wt (Figure 3E) embryos did not yield enough ectopic nuclei for cpHMM inference. However, their endogenous stripes followed the same bursting strategy observed in wild-type embryos, regardless of whether stripes were activated by wild-type or mutant enhancers (see SI Section: Complementary analysis of bursting parameters in Materials and methods). We inferred bursting parameters across regions of endogenous and ectopic nuclei in eveS1∆-eveS2Gt- embryos (eve stripe 1–2 and eve stripe 0), as they yielded sufficient ectopic eve-active nuclei to support cpHMM inference. As noted previously, these embryos feature an eve stripe 2 with nuclei of higher transcriptional output than wild-type embryos (compare Figure 2B and E), and a large region of ectopic expression towards the embryo anterior. Despite these differences in transcriptional output, bursting parameters in endogenous and ectopic eve-active nuclei in eveS1∆-eveS2Gt- embryos follow the same trends as wild-type embryos (Figure 3—figure supplement 2). In all regions–both endogenous and ectopic–enhancers increase transcription by increasing in kon and r, while koff remains largely constant (Figure 3F).
We performed an orthogonal cpHMM inference of bursting parameters by grouping nuclei in only two categories (endogenous and ectopic) (Figure 3—figure supplement 3), instead of grouping them according to their stripe, and we observed that this approach renders the same results (see SI Section: Complementary analysis of bursting parameters in Materials and Methods).
Taken together, our results show that all eve enhancers modulate their transcriptional output by increasing burst frequency (kon) and amplitude (r). koff, which shapes burst duration, remains largely constant, and shows a subtle drop as the mean MS2 fluorescence of nuclei increases. A wide range of transcriptional outputs result from these parameters. eve strategies of bursting control are robust to mutations on eve enhancers, and remain consistent in the presence of a myriad of inputs, including ectopic inputs different from those that shape the transcription of the seven canonical eve stripes.
Discussion
Over the last few years, the ability to infer bursting parameters from fixed (Little et al., 2013; Xu et al., 2015) and live-imaging (Lammers et al., 2020) data in embryos has revealed several commonalities and differences in the strategies employed by different enhancers to modulate bursting parameters and create patterns of gene expression (Berrocal et al., 2020; Zoller et al., 2018). For example, despite the different inputs that regulate the activity of eve enhancers, all of them modulate the expression of the seven canonical eve stripes by upregulating burst frequency (kon) and amplitude (r), while burst duration (koff–1) remains largely constant and shows only a minor increase in nuclei of high transcriptional output (Berrocal et al., 2020). Since the seven eve stripes are largely controlled by independent enhancers that respond to unique combinations of transcription factors, it was still unclear whether eve enhancers employ the same bursting strategy in ectopic regions, in the presence of trans-regulatory environments different from those that exist in their wild-type regions of expression.
Different bursting strategies between endogenous and ectopic regions of eve expression would suggest a selective pressure on eve enhancers that favors the observed bursting strategies at their canonical expression domains. On the other hand, unified bursting strategies in endogenous and ectopic regions point towards a common molecular mechanism, constrained by the biochemistry of enhancer-promoter interaction, which shapes the observed bursting parameters independent of changing trans-regulatory environments.
In this work, we compared bursting parameters (kon, koff, r) between endogenous and ectopic regions of eve expression to test between those two hypotheses. Specifically, we performed live imaging of eve-enhancer activity and bursting parameter inference in D. melanogaster embryos expressing wild-type and mutant versions of our BAC-based eveMS2 reporter system. Our observations provide evidence in favor of the second hypothesis, as we observe a unified strategy of bursting control wherever eve enhancers are active, regardless of the ectopic or endogenous inputs that regulate their activity. However, we acknowledge that our work cannot conclusively rule out the possibility that the observed strategies of bursting control may have been selected by evolution as the most optimal for the expression of the seven endogenous eve stripes. In this scenario, bursting control strategies would be conserved in ectopic expression regions as an evolutionarily neutral ‘passenger phenotype’. Regardless, the novelty of our current work lies in the insights derived from the comparative analysis of bursting control strategies between ectopic and endogenous eve expression regions, an aspect not addressed in Berrocal et al., 2020. In summary, despite changing trans-regulatory environments and mutations in enhancer sequence, eve enhancers act through a single promoter and upregulate transcriptional bursting in endogenous and ectopic expression regions. It is important to note that the modulation of burst frequency and amplitude is not the only possible bursting control strategy, and we emphasize that the unified strategies of eve bursting control described in this study do not necessarily apply to other genes. Indeed, (Zoller et al., 2018) observed that Drosophila gap genes, controlled by independent promoters and enhancers, modulate bursting through another common strategy; an increase in frequency and duration, while burst amplitude remains unchanged. A subsequent study by P.-T. Chen et al., 2023 found further evidence of a tight relationship between burst frequency and duration among gap genes. Consistent with our findings on eve bursting control, the authors observed that bursting control strategies for gap genes persist despite genetic perturbations. Furthermore, in a recent study, Syed et al., 2023 utilized a Hidden Markov Model to analyze live imaging data of transcription driven by snail enhancers. The study concludes that disrupting Dorsal binding sites on the snail minimal distal enhancer leads to a reduction in both the amplitude and duration of transcription bursting in fruit fly embryos. This work underscores the significance of enhancer-transcription factor interactions in shaping the bursting strategies of snail gene. These findings hint at an opportunity to classify enhancers and promoters in families whose members employ the same strategy of bursting control and rely on a common molecular mechanism to regulate their target genes.
In the light of our results, two molecular mechanisms coupled to enhancer activity could be behind the unified bursting strategies of eve enhancers. First, the observed common modulation of bursting parameters might result from general constraints imposed by the transcriptional machinery at enhancers or promoters. Previous work showed that topological dynamics of bacterial chromosomes brought by transcriptional activity shape bursting in bacteria (Chong et al., 2014); while histone acetylation of the circadian promoter Bmal1 modulates burst frequency in mammalian cells (Nicolas et al., 2018). Furthermore, Gorski et al., 2008 observed that the dynamics of RNA Pol I–subunit assembly affect transcriptional output. The dynamic nature of transcription factor ‘hubs’ (Mir et al., 2017; Tsai et al., 2017) in transcriptionally active enhancers of D. melanogaster embryos (Mir et al., 2018) may impact transcriptional bursting as well. The importance of modulating the concentration and availability of key transcription factors is emphasized by Hoppe et al., 2020. Their findings show that the naturally established concentration gradient of Bone Morphogenetic Protein (BMP) defines the bursting frequency of BMP target genes in fruit fly embryos. Another example that underscores the significance of transcription factor availability in shaping bursting strategies was illustrated by Zhao et al., 2024. Using optogenetic LEXY-mediated modulation of nuclear protein export (Niopek et al., 2016) in fruit fly embryos, this study found that the transcription factor Knirps represses the activity of the eve stripe 4+6 enhancer by gradually decreasing burst frequency until the locus sets into a fully reversible quiescent state. Systematic modulation of nuclear concentration through optogenetic LEXY for critical transcription factors such as Bicoid, Hunchback, Giant, Kruppel, and Zelda, will aid in fully elucidating the impact of transcription factor dynamics on eve bursting control strategies.
The second possibility is that the eve promoter, which is shared by all eve enhancers and distant regulatory elements, constrains the regulatory strategy of even-skipped. Recent studies using MS2 live imaging have described a fundamental role of core promoter elements, such as the TATA box, the initiator element, and the downstream core promoter element in shaping transcriptional bursting in genes of D. melanogaster embryos (Pimmett et al., 2021; Yokoshi et al., 2022). Furthermore, a survey of 17 genes in the actin family of the amoeba Dictyostelium discoideum (Tunnacliffe et al., 2018), featuring identical coding sequences but distinct promoters, revealed different bursting behaviors for each gene. These observations hint at a critical role of promoters in shaping bursting strategies. Further experiments, exploring the bursting strategies that result from swapping promoters in constructs carrying the eve enhancers could elucidate whether the eve promoter is responsible for establishing the eve regulatory strategy.
Both possibilities suggest that a molecular mechanism coupled to eve transcription restricts the landscape of bursting strategies available to eve enhancers. Our results indicate that eve bursting strategies are a fundamental property of enhancers and promoters—and not the result of changing trans-regulatory environments—and show that eve enhancers merely act as knobs, robust to mutations, that tune transcriptional output levels by modulating bursting through a largely fixed koff and shifting r and kon.
An ectopic pattern of particular interest is the novel eve stripe 0 brought by the deletion of the eve stripe 1 enhancer. This new stripe shows that mutations on existing eve enhancers can generate novel gene expression patterns through the same bursting strategies employed by the other eve stripes. Since expression patterns in embryonic development shape the formation and identity of animal body plans (Akam, 1983; Davidson, 2010; Lewis, 1978), the appearance of new expression patterns may constitute a critical driver of evolution (Rebeiz et al., 2011).
Materials and methods
DNA constructs and fly lines
We generated four reporter constructs based on a previously established Bacterial Artificial Chromosome (BAC) carrying the ~20 Kb DNA sequence around eve (Venken et al., 2006; Venken et al., 2009), and whose eve coding sequence has been replaced by an MS2::yellow transcriptional unit (Berrocal et al., 2020). We used wild-type eveMS2-BAC from Berrocal et al., 2020. The other three BAC constructs were derived from wild-type eveMS2-BAC. These constructs carried mutant versions of eve stripe 1 and eve stripe 2 enhancers. Vector Builder (https://en.vectorbuilder.com/) generated the mutant versions through ccdB-amp cassette mediated recombineering. These mutant BACs are available on Vector Builder’s website. SnapGene (.dna) files with eveMS2 BAC sequences are in the repository https://github.com/aberrocal/BurstingStrategies-eve, folder BurstingStrategies-eve/_DataSubmission/BACSequences/.
eveS1wt-eveS2Gt-
Request a detailed protocolBAC construct (Vector Builder-Service Proposal: P180328-1009dgs) contains a wild-type eve stripe 1 and a mutant version of eve stripe 2 enhancer with three Giant-binding sites deleted, as shown in Table 1 of Small et al., 1992. We chose to disrupt the three Gt-binding sites within the eve stripe 2 enhancer (Figure 2B) that had previously been tied to ectopic anterior expansion of eve stripe 2 expression when deleted in the context of the Minimal Regulatory Element of the eveS2 enhancer (eveS2-MRE; Small et al., 1992). eveS2-MRE is a 480 bp regulatory sequence within the eve stripe 2 enhancer (~2 kb total length) sufficient to drive the expression of eve stripe 2.
eveS1∆-eveS2Gt-
Request a detailed protocolBAC construct (Vector Builder-Service Proposal: P180614-1002pzr) has the eve stripe 1 enhancer, as defined by ChIP-seq data of the enhancer-associated protein Zelda (Harrison et al., 2011), replaced by a ccdB-amp cassette and eve stripe 2 enhancer replaced by a mutant version with three Giant binding sites deleted as described above.
eveS1∆-eveS2wt
Request a detailed protocolBAC construct (Vector Builder-Service Proposal: P190605-1001zkt) has eve stripe 1 enhancer replaced with a ccdB-amp cassette and a wild-type eve stripe 2. To sum up, we used the fly line carrying wild-type eveMS2-BAC from Berrocal et al., 2020 and we generated three new fly lines carrying genome integrations of the aforementioned constructs. The mutant versions of eveMS2-BAC used in this work were inserted in the genome via ϕC31 integrase-mediated recombination. Mutant constructs were either sent to BestGene Inc (eveS1wt-eveS2Gt-, eveS1∆-eveS2wt) for germline injection or injected in our laboratory (eveS1∆-eveS2Gt-). All constructs integrated into a ϕC31 AttP insertion site in chromosome 3 L (Bloomington stock #24871; landing site VK00033; cytological location 65B2).
Imaging
Request a detailed protocolWe crossed male flies from lines carrying eveMS2-BAC constructs (w-; +; MS2::yellow) and female flies carrying His::RFP and MCP::GFP fusion proteins (yw; His::RFP; MCP::GFP; Garcia et al., 2013). His::RFP allows for visualization of nuclei, MCP::GFP binds MS2 nascent transcripts to form fluorescent puncta at sites of nascent MS2 transcription. We set embryo-collection cages with ~30 male and ~100 female fruit flies, and collected offspring embryos after 1 hr 30 min. All movies in the same dataset were recorded within ~1 week. We mounted embryos on a slide for confocal imaging, as described in Berrocal et al., 2020; Bothma et al., 2014. Aging embryos for 1 hr 30 min allows us to capture the entire interval between the 14th synchronous cell cleavage and the beginning of gastrulation. We recorded a total of 22 live embryos as shown in Table 1. All imaging was done in a Zeiss-800 scanning-laser confocal microscope. Movies of embryonic development were captured under a 63 x oil objective, in windows of 202.8 µm x 50.7 µm, at pixel size of 0.2 µm, zoom 0.5 x. Movies were recorded in two channels, EGFP for MS2 signal, and TagRFP for His::RFP signal. Imaging parameters were 16 bits per pixel, scan mode frame, bidirectional scanning, scan speed 7, pixel dwelling 1.03 µs, laser scanning averaging 2, averaging method mean, averaging mode line, laser power EGFP 30 µW and TagRFP 7.5 µW, master gain in EGFP channel 550 V and in TagRFP channel 650 V, digital offset in both channels 0, digital gain in both channels 1, pinhole size 44 µm (1 Airy unit - 0.7 µm/section) at 63 x objective, laser filters EGFP:SP545 and TagRFP:LBF640. Data points consist of Z-stacks of 21 slices separated by intervals of 0.5 µm, to span a range of 10 µm across the Z axis. Z-stack mode full stack. Whole Z-stacks were recorded every 16.8 s (wild-type, eveS1wt-eveS2Gt-, eveS1∆-eveS2Gt-) and 19.5 s (eveS1∆-eveS2wt). The difference in time resolution between datasets does not impact our analysis, as the cpHMM analyzes interpolated data points at 20 s intervals. These parameters are based on the imaging protocol and settings in Berrocal et al., 2020. We stopped live imaging of individual embryos after 50 min into nuclear cycle 14, before the cell rearrangements of gastrulation, and took mid-sagittal and surface images of the whole embryo to localize our 202.8 µm x 50.7 µm window along the embryonic anterior-posterior axis. Raw data from confocal microscope imaging is publicly available in Zenodo (https://zenodo.org/, https://doi.org/10.5281/zenodo.7204096; see SI section: Data and Code) (Table 1).
Datasets and stripes.
We recorded 5 wild-type eveMS2-BAC (eveS1wt-eveS2wt) datasets, 6 eveS1wt-eveS2Gt- (eveS1wt_eveS2Gt), 5 eveS1∆-eveS2wt (eveS1Null_eveS2wt), and 6 eveS1∆-eveS2Gt- (eveS1Null_eveS2Gt) for a total of 22 datasets. Movies in every dataset capture between 3 and 6 stripes. Table 1 shows stripes captured in each dataset. Stripes in parentheses had few active nuclei (eveS0) or were not captured in their entirety (eveS4) and (eveS5). Asterisks indicate datasets used for stills in Figure 2.
| Wild-type datasets | Stripes Recorded |
|---|---|
| eveS1wt_eveS2wt_1 | eveS1, eveS2, eveS3, eveS4 |
| eveS1wt_eveS2wt_2 | eveS1, eveS2, eveS3, (eveS4) |
| eveS1wt_eveS2wt_3* | eveS1, eveS2, eveS3, eveS4 |
| eveS1wt_eveS2wt_4 | eveS1, eveS2, eveS3, eveS4 |
| eveS1wt_eveS2wt_5 | eveS1, eveS2, eveS3, eveS4, (eveS5) |
| eveS1wt-eveS2Gt- datasets | Stripes Recorded |
| eveS1wt_eveS2Gt_1 | eveS1, eveS1-2, eveS2, eveS3 |
| eveS1wt_eveS2Gt_2 | eveS1, eveS1-2, eveS2, eveS3, (eveS4) |
| eveS1wt_eveS2Gt_3 | eveS1, eveS1-2, eveS2, eveS3, eveS4 |
| eveS1wt_eveS2Gt_4 | eveS1, eveS1-2, eveS2, eveS3 |
| eveS1wt_eveS2Gt_5* | eveS1, eveS1-2, eveS2, eveS3, eveS4 |
| eveS1wt_eveS2Gt_6 | eveS1, eveS1-2, eveS2, eveS3, eveS4 |
| eveS1∆-eveS2wt datasets | Stripes Recorded |
| eveS1Null_eveS2wt_1 | (eveS0), eveS1, eveS2, eveS3, eveS4 |
| eveS1Null_eveS2wt_2* | eveS0, eveS1, eveS2, eveS3 |
| eveS1Null_eveS2wt_3 | eveS0, eveS1, eveS2, eveS3, (eveS4) |
| eveS1Null_eveS2wt_4 | (eveS0), eveS1, eveS2, eveS3, (eveS4) |
| eveS1Null_eveS2wt_5 | eveS0, eveS1, eveS2, eveS3 |
| eveS1∆-eveS2Gt- datasets | Stripes Recorded |
| eveS1Null_eveS2Gt_1 | eveS0, eveS1, eveS1-2, eveS2, eveS3, eveS4 |
| eveS1Null_eveS2Gt_2 | eveS0, eveS1, eveS1-2, eveS2, eveS3, eveS4 |
| eveS1Null_eveS2Gt_3 | eveS0, eveS1, eveS1-2, eveS2, eveS3, eveS4 |
| eveS1Null_eveS2Gt_4 | eveS0, eveS1, eveS1-2, eveS2, eveS3, eveS4 |
| eveS1Null_eveS2Gt_5* | eveS0, eveS1, eveS1-2, eveS2, eveS3 |
| eveS1Null_eveS2Gt_6 | eveS0, eveS1, eveS1-2, eveS2, eveS3 |
Segmentation and quantification of movies
Request a detailed protocolWe tracked MS2 foci from movies and segmented them using the MATLAB based analysis pipeline developed by Berrocal et al., 2020; Garcia et al., 2013; Lammers et al., 2020. Specifically, for segmentation of MS2/MCP::GFP foci across stacks on the Z-axis, we combined the MATLAB pipeline mentioned above with Fiji-Weka Segmentation 3D software, as described in Berrocal et al., 2020. The MATLAB/Fiji-Weka pipeline extracts the position of nuclei and the fluorescence intensity and position of individual MS2 foci over time. The final result of the MATLAB based analysis pipeline are CompiledParticles.mat files that contain the position of nuclei, as well as their MS2 fluorescence intensity over time (see Data and Code).
Assignment of eve-active nuclei to stripes
Request a detailed protocolWe manually segmented nuclei from eveS1∆-eveS2Gt- and eveS1wt-eveS2Gt- fly lines, as their stripes were not always clearly discernible. For these embryos, we assigned nuclei to individual stripes based on the position of stripes at 45 min into nc14, when they became separated from the background. The boundary between eve stripe 1–2 and eve stripe 2 in eveS1∆-eveS2Gt- embryos was set at 36% of embryo length, according to the kymograph of MS2 fluorescence over time. On the other hand, eveS1∆-eveS2wt and wild-type embryos showed defined stripes after 25 min into nc14. Thus, we used a MATLAB k-means clustering algorithm to dynamically assign eve-active nuclei to individual stripes, tracking nuclei by the accumulation of MS2 fluorescent output in windows of five-minutes. Nuclei active between 0 and 25 min into nc14 were assigned to stripes based on their position at 25 min into nc14. We generated movies of segmented MS2 spots assigned to individual stripes in windows of ~5 minutes. MATLAB scripts for manual and k-means-automated segmentation of stripes, as well as scripts to generate movies of segmented stripes are available in github (see Data and Code).
Generation of heatmaps in Figure 2 and Figure 2-Figure Supplement 1
Request a detailed protocolWe used traces of MS2 fluorescence intensity over time, which reflect transcriptional activity, to generate heatmap/kymographs of MS2 transcription datasets. We generated heatmaps (Figure 2, Figure 2—figure supplement 1) by collapsing data points from all embryos of the same genotype into a single kymograph plot. We started by adjusting the position of nuclei in each embryo relative to nuclei in other embryos of the same genotype. As we had assigned MS2 active nuclei to individual stripes, we measured the distance along the anterior-posterior axis from each MS2 focus to the center of its corresponding stripe. We inferred the position of pseudo-stripes formed by the combined data from all embryos of the same genotype. We calculated the position of pseudo-stripes along the anterior-posterior embryo axis by averaging the position of the center of stripes along the anterior-posterior axis in individual embryos of the same genotype. Finally, we assigned a position to all nuclei of the same genotype relative to pseudo-stripes by positioning them at the same distance from the center of pseudo-stripes as they were from the center of the stripe where they originated. We followed the same procedure to locate the position of inactive nuclei.
Labeling eve patterns as endogenous or ectopic
Request a detailed protocolTo compare the bursting parameters between endogenous and ectopic regions of eve activity, we segmented MS2-active nuclei and assigned them to individual regions that were deemed to be either endogenous or ectopic. We labeled regions as endogenous if their position overlapped within the boundaries of wild-type eve stripes (eve stripe 1, eve stripe 2, eve stripe 3, eve stripe 4); or as ectopic if their position overlapped with the inter-stripe region between eve stripe 1 and eve stripe 2 (eve stripe 1–2) or with the novel eve stripe 0 (~20% embryo length). All stripes in wild-type embryos were labeled as endogenous.
Selection of a three-state model of promoter activity and a compound Hidden Markov Model for inference of promoter states from MS2 fluorescent signal
Request a detailed protocolWe selected a three-state model of promoter activity (OFF, ON1, ON2) based on the following argument. Transcription in pre-gastrulating Drosophila embryos occurs after DNA replication, and sister chromatids remain paired. However, most of the time, paired MS2-tagged sister loci cannot be resolved independently using diffraction-limited microscopy (Lammers et al., 2020). Therefore, each fluorescent spot in our data results from the combined activity of two promoters, each of which, in the simplest possible model of transcriptional bursting, may be ON or OFF (Lammers et al., 2020). To account for this, the cpHMM infers three states from the observed MS2 data: OFF (both sister promoters inactive), ON1 (one sister promoter active), and ON2 (two sister promoters active). For ease of presentation, we aggregated ON1 and ON2 states into a single effective ON state, as we did in our previous work (Berrocal et al., 2020). This leads to an effective two-state model with one OFF and one ON state and three burst parameters: koff–1 (the burst duration), kon (the burst frequency), and r (the burst amplitude). kon is defined as the sum of the transition rates from OFF to any of the two active states described above: OFF → ON1 and OFF → ON2. koff is defined as the rate at which the system returns to the OFF state upon leaving it, which is described by the formula koff–1 = ( - 1) kon–1, where is the fraction of time the system spends in the OFF state. koff is the inverse of mean burst duration. r is defined by the average of the rates of transcription initiation in the two ON states (r1 and r2) weighted by the fraction of the time that the system spends on each state (p1 and p2) as described by the formula r = (Lammers et al., 2020). The outputs of the three state model of promoter activity (kon, koff, and r) were used for downstream analyses.
The three-state model of promoter activity is the simplest model compatible with our current understanding of transcription at the eve locus in early fruit fly embryos. However, we do not dismiss the possibility that more complex processes, not captured by our model, define eve transcription. Promoters, for instance, may exhibit more than two states of activity, beyond a simple ON and OFF mechanism. Nevertheless, as pointed out by Lammers et al., 2020 - SI Section: G. the cross-validation of cpHMM inference sensitivities between different model schemes (two, three, or multiple state Hidden Markov Models) do not yield consistent results regarding on which one is more accurate; and for the time being, there is no alternative to a HMM for inference of promoter states from MS2/PP7 fluorescence signals obtained using laser-scanning confocal microscopy (Lammers et al., 2020; Syed et al., 2023; although other approaches exist using state-of-the-art microscopy and deconvolution algorithms to improve signal-to-noise ratio). Furthermore, orthogonal approaches to quantify transcription that rely on static methods, such as smFISH, have a limited ability to capture temporal dynamics. Due to these considerations, we selected a HMM based on an effective two-state model (derived from a three-state model) of promoter activity to describe our live MS2 imaging data.
Inference of bursting parameters
Request a detailed protocolWe used a cpHMM approach (Lammers et al., 2020) to extract average bursting parameters (kon, koff, r) from different sets of MS2-active nuclei. We input MS2 fluorescent traces over time from these sets into the cpHMM. Specifically, we combined nuclei from same-genotype embryos, sorted them by stripe and distributed them across bins of varying fluorescence. To ensure reliable inference, we enforced each bin to contain ~40 nuclei, equivalent to ~2500 time points at a 20 s resolution (Lammers et al., 2020). The number of bins was determined by the amount of data available (Table 2).
Binning by stripe.
We pooled together nuclei from all embryos per dataset, sorted them by the stripe where they were located and distributed them in bins of varying fluorescence. Each bin contains ~40 nuclei (~2,500 time points). E.g., all nuclei in eve stripe 1 (eveS1) from the five eve wild-type embryos in our dataset were assigned to 3 bins according to their mean MS2 fluorescence, as each bin must contain ~40 nuclei, or ~2,500 data points, for input into the cpHMM.
| Wild-type - Stripes | Number of bins |
|---|---|
| eveS1 | 3 |
| eveS2 | 4 |
| eveS3 | 3 |
| eveS4 | 3 |
| eveS5 | 0 |
| eveS1wt-eveS2Gt- - Stripes | Number of bins |
| eveS1 | 4 |
| eveS1-2 | 0 |
| eveS2 | 5 |
| eveS3 | 4 |
| eveS4 | 2 |
| eveS1∆-eveS2wt - Stripes | Number of bins |
| eveS0 | 0 |
| eveS1 | 2 |
| eveS2 | 4 |
| eveS3 | 3 |
| eveS4 | 1 |
| eveS1∆-eveS2Gt- - Stripes | Number of bins |
| eveS0 | 3 |
| eveS1 | 4 |
| eveS1-2 | 3 |
| eveS2 | 6 |
| eveS3 | 5 |
| eveS4 | 3 |
Wild-type embryos yielded sufficient nuclei to support the cpHMM inference of bursting parameters for various endogenous stripes (eve stripe 1, 2, 3, 4). eveS1wt-eveS2Gt- and eveS1∆-eveS2wt did not yield enough ectopically active nuclei for cpHMM analysis (eve stripe 1–2 in eveS1wt-eveS2Gt-; eve stripe 0 in eveS1∆-eveS2wt). These fly lines did exhibit endogenous eve stripes with enough active-nuclei for further analysis on the cpHMM (eve stripe 1, 2, 3, and 4 in eveS1wt-eveS2Gt-; eve stripe 1, 2, and 3 in eveS1∆-eveS2wt). eveS1∆-eveS2Gt- embryos did yield sufficient eve-active nuclei (297 nuclei) to support cpHMM inference of the bursting parameters of ectopic eve stripe 1–2 and eve stripe 0. It also resulted in enough active nuclei for the cpHMM inference of bursting parameters of endogenous stripes (eve stripe 1, 2, 3, and 4).
The output of the effective two-state cpHMM described above are the bursting parameters (kon, koff, r) for each set of nuclei input into the model. Thus, Figure 3, Figure 3—figure supplement 2 are plots of mean kon, koff, r, and their standard deviations σkon, σkoff, σr, computed from sets of nuclei binned by stripe. For Figure 3—figure supplement 3, we followed a similar approach, but grouping active nuclei by their endogenous or ectopic location. Nuclei grouped in endogenous and ectopic categories were distributed across 6–13 bins of increasing fluorescence (Table 3). Their mean kon, koff, r, and standard deviations, σkon, σkoff, σr were plotted in Figure 3—figure supplement 3.
Binning by endogenous/ectopic.
We pooled together nuclei from all embryos per dataset, sorted them by endogenous or ectopic, according to whether the stripe where they were located was deemed endogenous or ectopic, and distributed them in bins of varying fluorescence. Each bin contains ~40 nuclei (~2500 time points). E.g. All endogenous nuclei in the 5 eve wild-type embryos were distributed among 11 bins of increasing MS2 fluorescence. Some datasets have their ectopic bin empty, as they had less than ~40 active nuclei in their ectopic regions.
| Wild-type | Number of Bins |
|---|---|
| Ectopic | 0 |
| Endogenous | 11 |
| eveS1wt-eveS2Gt- | Number of Bins |
| Ectopic | 0 |
| Endogenous | 13 |
| eveS1∆-eveS2wt | Number of Bins |
| Ectopic | 0 |
| Endogenous | 7 |
| eveS1∆-eveS2Gt- | Number of Bins |
| Ectopic | 6 |
| Endogenous | 11 |
Data and code
Request a detailed protocolRaw data, Movies, and CompiledParticles files are stored in the Zenodo dataset ‘Unified bursting strategies in ectopic and endogenous even-skipped expression patterns - Supplemental Data’ (https://doi.org/10.5281/zenodo.7204096; Berrocal et al., 2023). Specific paths in this dataset are listed below. Raw confocal-imaging data from embryos of each of the genotypes used in this work are located in [Genotype]_rawData/[Date]/[Dataset] as czi files (Zeiss file format) of sequential Z-stacks recorded over two channels, and whole embryo stills, as described above. Maximum Z-projection movies of all recorded embryos are in Movies/[Genotype]/Composite. Movies of MS2-foci assigned to stripes are in Movies/[Genotype]/Segmentation. The outcome of Garcia et al., 2013 MATLAB pipeline to analyze MS2 data from each embryo are mat files named CompiledParticles, they are stored in the folder CompiledParticles/[Genotype].
MATLAB scripts and data for this analysis are stored in the github repository https://github.com/aberrocal/BurstingStrategies-eve. The code for the segmentation of our live imaging data of eve transcription in embryonic development is in BurstingStrategies-eve/_DataSubmission/DataSheetsAndCode/StripeSegmentation/. We generated csv files containing the position of active and inactive nuclei over time for each of four genotypes (see BurstingStrategies-eve/_DataSubmission/DataSheetsAndCode/Heatmaps/singleTraceFits_Heatmaps/). In these files, active nuclei have fluorescence values associated with each time point. These datasets also contain the promoter state of active nuclei at each time point. We considered three promoter states: 1=OFF, 2=one sister promoter ON (ON1), and 3=two sister promoters ON (ON2); see SI section: Inference of bursting parameters in Materials and methods. The heatmaps in this work (Figure 2, Figure 2—figure supplement 1) were generated with MATLAB scripts and datasets in BurstingStrategies-eve/_DataSubmission/DataSheetsAndCode/Heatmaps/. We generated mat files (compiledResults_[Stripe/ectopicFlag].mat) that contain mean values of kon (frequency), koff–1 (duration), r (amplitude), their standard deviations, and mean fluorescence bin values. compiledResults_Stripe.mat files and scripts to generate Figure 3, Figure 3—figure supplement 2 are sorted by genotype in BurstingStrategies-eve/_DataSubmission/DataSheetsAndCode/KineticsPlotStripes_Color/. compiledResults_ectopicFlag.mat and scripts to generate Figure 3—figure supplement 3 are sorted by genotype in BurstingStrategies-eve/_DataSubmission/DataSheetsAndCode/KineticsPlotsEndogenousEctopic/. Data to generate Tables 2 and 3 is located in BurstingStrategies-eve/_DataSubmission/DataSheetsAndCode/BinStats/particle_counts/. Data sheets with detailed features of individual data points (identity and position of nuclei and MS2 foci; MS2 fluorescence; cpHMM-inference of fluorescence; cpHMM-inferred promoter state) are located in BurstingStrategies-eve/_DataSubmission/DataSheetsAndCode/BinStats/singleTraceFits/. Adobe Illustrator ai, eps, and png files for all figures are stored in BurstingStrategies-eve/_DataSubmission/Figures/.
Materials availability statement
Request a detailed protocolFly line expressing wild-type eveMS2-BAC is available at Bloomington Drosophila Stock center (stock #92368). Wild-type and mutant eveMS2-BAC constructs can be obtained through Vector Builder, as described in ‘DNA constructs and fly lines’, or by requesting them from the corresponding authors. For ChIP-seq data of enhancer associated-Zelda refer to ‘Data Availability’ section of Harrison et al., 2011. Details on sample preparation for confocal imaging are provided in the ‘Embryo collection and mounting’ section of Berrocal et al., 2020. Raw confocal imaging data, movies, and compiled particles files are stored in Zenodo, as described in ‘Data and Code’. MATLAB code to segment MS2 signals in fruit fly embryos and generate compiled particles files, as described in ‘Segmentation and quantification of movies’, is publicly available in https://github.com/GarciaLab/mRNADynamics (Reimer and Garcia, 2022). For details on cpHMM scripts and methods refer to ‘Data Availability’ section in Lammers et al., 2020. DNA sequences, figures, and MATLAB code for data analysis are stored in https://github.com/aberrocal/BurstingStrategies-eve, as described in ‘Data and Code’.
Appendix 1
Supplemental Information
Complementary analysis of bursting parametersComplementary Analysis of Bursting Parameters
Bursting parameters in endogenous stripes controlled by mutant enhancers
Some stripes in this work are driven by mutant eve enhancers. We found that mutated enhancers modulate transcriptional output of endogenous stripes through the same mechanism as their wild-type counterparts: an increase in kon and r, while koff remains largely constant (Figure 3—figure supplement 2). In eveS1wt-eveS2Gt- embryos (Figure 3—figure supplement 2C), eve stripe 2 is driven by a mutant eve stripe 2 enhancer. In eveS1∆-eveS2wt embryos (Figure 3—figure supplement 2D), eve stripe 1 is active in the absence of eve stripe 1 enhancer, perhaps due to the activity of the late element. In eveS1∆-eveS2Gt- embryos (Figure 3—figure supplement 2E), eve stripe 2 is driven by a mutant eve stripe 2 enhancer and eve stripe 1 is active in the absence of eve stripe 1 enhancer. In all cases, our findings support the hypothesis that eve-regulatory elements employ a unified strategy to modulate transcriptional output. Bursting parameters of eve stripe 1 in embryos with a deleted eve stripe 1 enhancer (eveS1∆-eveS2wt; eveS1∆-eveS2Gt-) are of particular interest, as this expression is most likely activated by the eve late element. If this is the case, the eve late element would modulate transcriptional output through the same mechanism as the other enhancers, further underlining the unity of regulatory strategies across different eve-regulatory elements.
Comparison of bursting parameters between sets of nuclei grouped in endogenous and ectopic categories
We computed the bursting parameters of 3–6 bins per stripe (Table 2), depending on the amount of data obtained (see SI: Figure 3—figure supplement 1 and Inference of bursting parameters in Materials and methods). To rule out the possibility that the observed kon, koff, and r trends were skewed by the small number of bins, we aimed to redo our analysis with more data points per category (endogenous and ectopic), as a way to contrast bursting parameters between whole endogenous and ectopic regions and examine the bursting parameters trends that result from having 6–13 bins per category (Table 3).
We pooled together all nuclei from eveS1∆-eveS2Gt- embryos into endogenous (eve stripe 1, eve stripe 2, eve stripe 3, eve stripe 4) and ectopic sets (eve stripe 0, eve inter-stripe 1–2), and binned them by their mean MS2 fluorescence output to infer and compare their bursting parameters. We did the same analysis in wild-type, eveS1wt-eveS2Gt-, and eveS1∆-eveS2wt embryos. We contrasted the bursting parameters of ectopic nuclei from eveS1∆-eveS2Gt- embryos against sets of endogenous nuclei from eveS1∆-eveS2Gt- eveS1wt-eveS2Gt-, eveS1∆-eveS2wt, and wild-type embryos (Figure 3—figure supplement 3) and observed that all of them follow the same bursting strategy. Ectopic nuclei from eveS1∆-eveS2Gt- embryos boost transcriptional output through an increase in average kon (Figure 3—figure supplement 3B) and r (Figure 3—figure supplement 3D), while koff remains largely the same, with only a minor drop at high mean MS2 fluorescence values (Figure 3—figure supplement 3C). The bursting parameters of endogenous nuclei from all the genotypes in this work follow the same trend.
Data availability
Raw data, Movies, and CompiledParticles files are stored in Zenodo (https://doi.org/10.5281/zenodo.7204096). Specific paths in this dataset are listed in Data and Code section of this article. MATLAB scripts and processed data for this analysis are stored in github (https://github.com/aberrocal/BurstingStrategies-eve, copy archived at Berrocal et al., 2024), as described in Data and Code section of this article.
-
ZenodoUnified bursting strategies in ectopic and endogenous even-skipped expression patterns - Supplemental Data.https://doi.org/10.5281/zenodo.7204096
-
NCBI Gene Expression OmnibusID GSE30757. Zelda binding in the early Drosophila melanogaster embryo marks regions subsequently activated at the maternal-to-zygotic transition.
References
-
SoftwareBurstingStrategies-eve, version swh:1:rev:b4cde1182576b63b21f91dccf8744410a763e046Software Heritage.
-
Dynamic interplay between enhancer-promoter topology and gene activityNature Genetics 50:1296–1303.https://doi.org/10.1038/s41588-018-0175-z
-
Transcriptional pulsing of a developmental geneCurrent Biology 16:1018–1025.https://doi.org/10.1016/j.cub.2006.03.092
-
BookThe Regulatory Genome: Gene Regulatory Networks In Development And EvolutionElsevier.
-
Measuring transcription dynamics in living cells using fluctuation analysisMethods in Molecular Biology 1042:47–60.https://doi.org/10.1007/978-1-62703-526-2_4
-
A mutation in the Drosophila melanogaster eve stripe 2 minimal enhancer is buffered by flanking sequencesG3: Genes, Genomes, Genetics 10:4473–4482.https://doi.org/10.1534/g3.120.401777
-
Dense Bicoid hubs accentuate binding along the morphogen gradientGenes & Development 31:1784–1794.https://doi.org/10.1101/gad.305078.117
-
Optogenetic control of nuclear protein exportNature Communications 7:10624.https://doi.org/10.1038/ncomms10624
-
Markovian modeling of gene-product synthesisTheoretical Population Biology 48:222–234.https://doi.org/10.1006/tpbi.1995.1027
-
Arthropod segmentation: beyond the Drosophila paradigmNature Reviews. Genetics 6:905–916.https://doi.org/10.1038/nrg1724
-
HIV promoter integration site primarily modulates transcriptional burst size rather than frequencyPLOS Computational Biology 6:e1000952.https://doi.org/10.1371/journal.pcbi.1000952
-
Regulation of even-skipped stripe 2 in the Drosophila embryoThe EMBO Journal 11:4047–4057.https://doi.org/10.1002/j.1460-2075.1992.tb05498.x
-
Single-RNA counting reveals alternative modes of gene expression in yeastNature Structural & Molecular Biology 15:1263–1271.https://doi.org/10.1038/nsmb.1514
Article and author information
Author details
Funding
Howard Hughes Medical Institute
- Michael B Eisen
NIH R01 Award (R01GM139913)
- Hernan G Garcia
NIH Genomics and Computational Biology Training Grant (5T32HG000047-18)
- Nicholas C Lammers
Winkler Scholar Faculty Award in the Biological Sciences
- Hernan G Garcia
Koret-UC Berkeley-Tel Aviv University Initiative in Computational Biology and Bioinformatics
- Hernan G Garcia
Chan Zuckerberg Biohub-San Francisco Investigator
- Hernan G Garcia
University of California Institute for Mexico and the United States (UC MEXUS) Doctoral Fellowship
- Augusto Berrocal
NIH R01 Award (R01GM152815)
- Hernan G Garcia
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We acknowledge Edward Pym and Michael Stadler for their insightful comments on the manuscript, as well as all the members of the Eisen Lab and the Garcia Lab for their valuable contributions to our discussions.
Version history
- Preprint posted:
- Sent for peer review:
- Reviewed Preprint version 1:
- Reviewed Preprint version 2:
- Version of Record published:
- Version of Record updated:
Cite all versions
You can cite all versions using the DOI https://doi.org/10.7554/eLife.88671. This DOI represents all versions, and will always resolve to the latest one.
Copyright
© 2023, Berrocal et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,115
- views
-
- 42
- downloads
-
- 3
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Chromosomes and Gene Expression
- Genetics and Genomics
Models of nuclear genome organization often propose a binary division into active versus inactive compartments yet typically overlook nuclear bodies. Here, we integrated analysis of sequencing and image-based data to compare genome organization in four human cell types relative to three different nuclear locales: the nuclear lamina, nuclear speckles, and nucleoli. Although gene expression correlates mostly with nuclear speckle proximity, DNA replication timing correlates with proximity to multiple nuclear locales. Speckle attachment regions emerge as DNA replication initiation zones whose replication timing and gene composition vary with their attachment frequency. Most facultative LADs retain a partially repressed state as iLADs, despite their positioning in the nuclear interior. Knock out of two lamina proteins, Lamin A and LBR, causes a shift of H3K9me3-enriched LADs from lamina to nucleolus, and a reciprocal relocation of H3K27me3-enriched partially repressed iLADs from nucleolus to lamina. Thus, these partially repressed iLADs appear to compete with LADs for nuclear lamina attachment with consequences for replication timing. The nuclear organization in adherent cells is polarized with nuclear bodies and genomic regions segregating both radially and relative to the equatorial plane. Together, our results underscore the importance of considering genome organization relative to nuclear locales for a more complete understanding of the spatial and functional organization of the human genome.
-
- Chromosomes and Gene Expression
- Genetics and Genomics
Among the major classes of RNAs in the cell, tRNAs remain the most difficult to characterize via deep sequencing approaches, as tRNA structure and nucleotide modifications can each interfere with cDNA synthesis by commonly-used reverse transcriptases (RTs). Here, we benchmark a recently-developed RNA cloning protocol, termed Ordered Two-Template Relay (OTTR), to characterize intact tRNAs and tRNA fragments in budding yeast and in mouse tissues. We show that OTTR successfully captures both full-length tRNAs and tRNA fragments in budding yeast and in mouse reproductive tissues without any prior enzymatic treatment, and that tRNA cloning efficiency can be further enhanced via AlkB-mediated demethylation of modified nucleotides. As with other recent tRNA cloning protocols, we find that a subset of nucleotide modifications leave misincorporation signatures in OTTR datasets, enabling their detection without any additional protocol steps. Focusing on tRNA cleavage products, we compare OTTR with several standard small RNA-Seq protocols, finding that OTTR provides the most accurate picture of tRNA fragment levels by comparison to "ground truth" Northern blots. Applying this protocol to mature mouse spermatozoa, our data dramatically alter our understanding of the small RNA cargo of mature mammalian sperm, revealing a far more complex population of tRNA fragments - including both 5′ and 3′ tRNA halves derived from the majority of tRNAs – than previously appreciated. Taken together, our data confirm the superior performance of OTTR to commercial protocols in analysis of tRNA fragments, and force a reappraisal of potential epigenetic functions of the sperm small RNA payload.






