Inhibition of the Notch signal transducer CSL by Pkc53E-mediated phosphorylation to fend off parasitic immune challenge in Drosophila
eLife Assessment
This valuable study focuses on the regulation of Notch signaling during the immune response in Drosophila. The authors provide solid evidence in support of roles for Su(H) and Pkc53E-induced phosphorylation in Drosophila immunity. The work will be of interest to colleagues in immunity and receptor signaling.
https://doi.org/10.7554/eLife.89582.3.sa0Valuable: Findings that have theoretical or practical implications for a subfield
- Landmark
- Fundamental
- Important
- Valuable
- Useful
Solid: Methods, data and analyses broadly support the claims with only minor weaknesses
- Exceptional
- Compelling
- Convincing
- Solid
- Incomplete
- Inadequate
During the peer-review process the editor and reviewers write an eLife Assessment that summarises the significance of the findings reported in the article (on a scale ranging from landmark to useful) and the strength of the evidence (on a scale ranging from exceptional to inadequate). Learn more about eLife Assessments
Abstract
Notch signalling activity regulates hematopoiesis in Drosophila and vertebrates alike. Parasitoid wasp infestation of Drosophila larvae, however, requires a timely downregulation of Notch activity to allow the formation of encapsulation-active blood cells. Here, we show that the Drosophila CSL transcription factor Suppressor of Hairless [Su(H)] is phosphorylated at Serine 269 in response to parasitoid wasp infestation. As this phosphorylation interferes with the DNA binding of Su(H), it reversibly precludes its activity. Accordingly, phospho-deficient Su(H)S269A mutants are immune-compromised. A screen for kinases involved in Su(H) phosphorylation identified Pkc53E, required for normal hematopoiesis as well as for parasitoid immune response. Genetic and molecular interactions support the specificity of the Su(H)-Pkc53E relationship. Moreover, phorbol ester treatment inhibits Su(H) activity in vivo and in human cell culture. We conclude that Pkc53E targets Su(H) during parasitic wasp infestation, thereby remodelling the blood cell population required for wasp egg encapsulation.
Introduction
Drosophila melanogaster harbours a sophisticated cellular immune system to fight invaders. The larval hematopoietic system comprises a circulating and sessile compartment of embryonic origin and the developing larval lymph gland, constituting a hematopoietic organ. The circulating and sessile compartment consists primarily of macrophage-like plasmatocytes, plus a small number of crystal cells involved in wound healing and melanisation responses to neutralise pathogens. Both cell types differentiate from hemocyte precursors within the lymph gland as well, and they are released during pupal stages to serve the adult with immune cells. Finally, lamellocytes represent the third blood cell type in Drosophila. While merely absent in healthy animals, their formation is induced within hours of wasp infestation or wounding (reviewed in Banerjee et al., 2019; Letourneau et al., 2016; Hultmark and Andó, 2022). In fact, parasitism by endo-parasitoid wasps represents one of the most severe naturally occurring immune challenges to Drosophila, invariably causing death if not defended off properly. Parasitoid wasps deposit their egg into the live Drosophila larva, where it develops into the next wasp generation egressing from the pupa instead of a fly. Hence, wasp infestation is a life-threatening challenge to the Drosophila host, demanding an immediate immune response to overwhelm the parasite. This involves a massive increase in circulating hemocytes, and most energy resources are devoted to fight the invader. Here, lamellocytes play a critical role: they encapsulate the wasp egg and, by expressing prophenoloxidase, induce a melanisation reaction retarding further wasp development (Dudzic et al., 2015; reviewed in: Banerjee et al., 2019; Letourneau et al., 2016; Hultmark and Andó, 2022).
Wasp infestation substantially remodels the composition of the Drosophila hemocyte population (Cattenoz et al., 2020; Cho et al., 2020; Tattikota et al., 2020; reviewed in Csordás et al., 2021). There is a vast increase in plasmatocytes and intermediate precursors in both hematopoietic compartments, from which lamellocyte differentiate. This process requires the combined regulatory input of several pathways, including JAK/STAT, JNK, Toll, Notch, EGFR, and INR pathways, which in fact regulate blood cell homeostasis in general (reviewed in Banerjee et al., 2019; Letourneau et al., 2016; Csordás et al., 2021). Simultaneous to the massive expansion of lamellocytes, crystal cells are significantly reduced (Crozatier et al., 2004; Krzemien et al., 2010; Ferguson and Martinez-Agosto, 2014; Cattenoz et al., 2020; Cho et al., 2020; Tattikota et al., 2020; reviewed in Csordás et al., 2021). Crystal cells are generated both in the larval lymph gland and by transdifferentiation from plasmatocytes in the sessile compartment. Their formation, differentiation, and survival strictly depend on Notch signalling activity (reviewed in Banerjee et al., 2019; Csordás et al., 2021; Hultmark and Andó, 2022). Lamellocyte and crystal cell lineages are mutually exclusive. Accordingly, while promoting crystal cell fate, Notch activity inhibits differentiation of lamellocytes within the lymph gland, however, is reduced upon wasp infestation (Small et al., 2014). Lamellocyte induction involves the formation and sensing of reactive oxygen species triggered by wasp egg injection (Nappi et al., 1995; Sinenko et al., 2011; Small et al., 2014; Louradour et al., 2017; reviewed in Banerjee et al., 2019; Csordás et al., 2021). The molecular mechanism underlying the simultaneous crystal cell fate inhibition, however, is less well understood. Obviously, an effective and timely downregulation of Notch activity is required to fend off parasitic wasps.
The Notch pathway is highly conserved between invertebrates and vertebrates, where it regulates numerous cell fate decisions. Notch signals are transduced by CSL-type proteins (abbreviation of human CBF1/RBPJ, Drosophila Suppressor of Hairless [Su(H)], and worm Lag1), that bind the DNA to direct transcriptional activity of Notch target genes by help of recruited cofactors (reviewed in Giaimo et al., 2021; Kopan and Ilagan, 2009; Siebel and Lendahl, 2017). In the absence of Notch signals, however, CSL together with co-repressors silences Notch target genes, thereby acting as a molecular switch (Borggrefe and Oswald, 2009). Hence, CSL is central to Notch pathway activity as no signal transduction can occur in its absence or in the instance of a lack of DNA binding. Earlier, we observed Su(H) phosphorylation at Serine 269 in cultured Drosophila Schneider S2 cells (Nagel et al., 2017). Of note, Schneider S2 cells have hemocyte characteristics (Cherbas et al., 2011; Schneider, 1972; Terriente-Felix et al., 2013). As a consequence of the negative charge conferred by the phosphorylation at Serine 269, Su(H) loses its affinity to the DNA, and without its DNA-binding ability, also its function as a transcriptional regulator (Nagel et al., 2017). Accordingly, a phospho-mimetic Su(H)S269D mutant behaved like a Su(H) loss-of-function mutant in all respects. In contrast, the phospho-deficient Su(H)S269A variant, appeared wild-type at the first glance, indicating some tissue specificity of this regulatory mechanism. In fact, the Su(H)S269A allele displayed a gain of Notch activity particularly during embryonic and larval hematopoiesis with increased numbers of crystal cells in both hematopoietic compartments (Frankenreiter et al., 2021). Moreover, we found that the general Notch antagonist Hairless is not involved in constraining crystal cell numbers, suggesting that in the context of blood cell homeostasis Notch activity is regulated by the phosphorylation of Su(H) (Maier, 2006; Frankenreiter et al., 2021). As the same set of genes affect blood cell maintenance and differentiation in homeostasis and upon immune challenge (Cho et al., 2020; Tattikota et al., 2020), obviously phospho-mediated downregulation of Notch activity might also occur in response to parasitoid wasp infestation, allowing the formation of encapsulation-active lamellocytes at the expense of crystal cells. Briefly, phosphorylation of Su(H) is an elegant mechanism to transiently curb Notch activity in the context of immune responses.
In this work, we followed the hypothesis that after wasp infestation, a specific kinase might be activated to phosphorylate Su(H) thereby allowing an adequate immune response. In this case, the phospho-deficient Su(H)S269A allele should be immune-compromised, as it cannot respond to phosphorylation, i.e., remaining active even upon parasitism. Indeed, Su(H)S269A displayed an increased sensitivity towards parasitoid wasp infestation accompanied by an increase of crystal cells at the expense of lamellocytes. Accordingly, phosphorylation of Su(H) protein was detected in infested wild-type larvae but not in the Su(H)S269A mutant. In a screen for kinases regulating this process, Pkc53E, the homologue of human PKCα, was identified as an important player. In agreement with a role in blood cell homeostasis, a Pkc53EΔ28 null mutant displayed increased crystal cell numbers. Moreover, genetic and molecular interactions between Su(H) and Pkc53E support the specificity of their relationship. Finally, Pkc53EΔ28 was impaired in its immune response to wasp infestation as well. Together, these data show that Su(H) is a target of Pkc53E during parasitic wasp infestation, inducing phosphorylation and subsequent downregulation of Su(H) activity to allow the mass production of lamellocytes required for wasp defense.
Results
Impaired immune response to parasitoid wasps in the phospho-deficient Su(H)S269A mutant
Wasp infestation alters the course of hematopoiesis, as lamellocyte differentiation is massively increased at the expense of crystal cells. This process requires a timely attenuation of Notch activity that may be implemented by the phosphorylation of Su(H) at Serine 269 (S269). To test this model, we exposed control larvae and larvae of the phospho-deficient Su(H)S269A variant to the parasitoid wasp Leptopilina boulardi (L. boulardi) and analysed the consequences on blood cell homeostasis around 44 hr post-infection, i.e., before lymph gland histolysis. To exclude any influence of the engineered genomic background, we used Su(H)gwt for comparison, carrying a genomic wild-type construct in place of the mutant (Praxenthaler et al., 2017; Frankenreiter et al., 2021).
Earlier we noted an excess of crystal cells in the phospho-deficient Su(H)S269A allele, which we interpret as a gain of Notch activity in consequence of the inability to be downregulated by Su(H) phosphorylation (Frankenreiter et al., 2021; Figure 1A and B). In agreement with this hypothesis, RNAi-mediated downregulation of Notch in hemocytes (hml::N-RNAi) resulted in a near-complete loss of crystal cells. This Notch loss-of-function phenotype was epistatic to the Su(H)S269A phenotype, i.e., the excess of crystal cells characterising Su(H)S269A was no longer observed in the combination with N-RNAi, demonstrating that Notch acted upstream of Su(H) as expected (Figure 1—figure supplement 1). The slightly elevated numbers in the Su(H)S269A background compared to the control, however, may be due to the enlarged anlagen in the embryo unaffected by hml::N-RNAi (Frankenreiter et al., 2021). Total hemocyte numbers were slightly, albeit not significantly increased in Su(H)S269A compared to the Su(H)gwt control, and correspondingly, hemocyte numbers were somewhat lowered in the Su(H)S269D allele (Figure 1—figure supplement 1). This is in line with earlier observations of unchanged plasmatocyte counts in N or Su(H) mutants relative to control (Duvic et al., 2002). In response to wasp infestations, however, crystal cell numbers should drop to allow the formation of lamellocytes (Small et al., 2014; Csordás et al., 2021). Indeed in the Su(H)gwt control, both the sessile crystal cells and those within the larval lymph glands were significantly lessened in response to wasp infestation (Figure 1A and B). In contrast, the higher crystal cell numbers in the Su(H)S269A mutant larvae dropped to control level, demonstrating the impairment of the mutant to detect this immune challenge or to respond to it (Figure 1A and B). Total hemocyte numbers, however, were similar between the genotypes independent of wasp infestation (Figure 1—figure supplement 1).
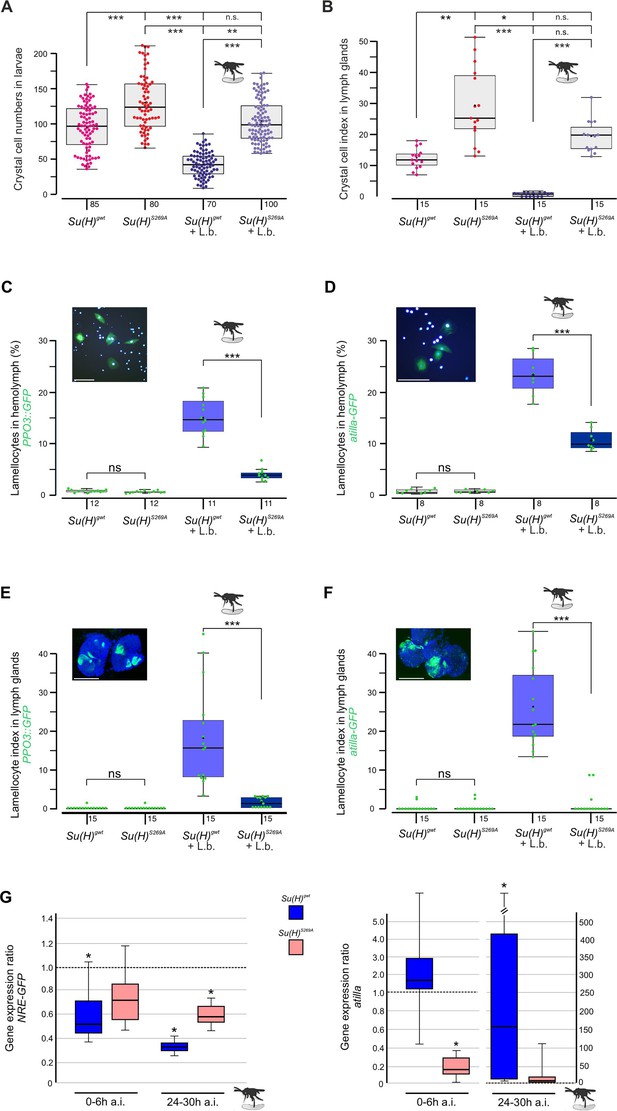
Su(H)S269A mutants are compromised in their response to parasitoid wasp infestation.
(A) Quantification of melanised crystal cells from the last two segments of Su(H)gwt and Su(H)S269A larvae with and without wasp infestation as indicated. In the control, wasp parasitism causes crystal cell numbers to drop to a level of about 50%, whereas in Su(H)S269A mutants the number settles at the uninfested Su(H)gwt level. Each dot represents one analysed larva (n=70–100 as indicated). (B) Crystal cell index in larval lymph glands is given as ratio of Hnt-positive crystal cells per 1° lobe relative to the size of the lobe. Each point represents one analysed lobus (n=15). (A, B) Statistical analyses with Kruskal-Wallis test, followed by Dunn’s test with ***p<0.001, **p<0.01, *p<0.05, ns (not significant p≥0.05). (C–F) Quantification of larval lamellocytes in the circulating hemolymph (C, D) or in lymph glands (E, F) before and after wasp infestation in Su(H)gwt versus Su(H)S269A. Lamellocytes were marked with either PPO3-Gal4::UAS-GFP (C, E) or atilla-GFP (D, F) as indicated. (C, D) The fraction of GFP-labelled lamellocytes of the total number of DAPI-labelled blood cells isolated from hemolymph is given; each dot represents 10 pooled larvae (n=8–10 as shown). Representative image of labelled control hemolymph is shown above (DAPI-labelled nuclei in light blue, GFP in green). Scale bars, 50 µm. (E, F) Lamellocyte index is given as number of GFP-labelled lamellocytes per area in the 1° lobe of the lymph gland. Each dot represents the lamellocyte index of one lobus (n=15). Representative Su(H)gwt lymph glands after wasp infestation are shown, co-stained for nuclear Pzg (in blue). Scale bars, 100 µm. Statistical analyses with unpaired Student’s t-test; only significant differences are indicated (***p<0.001). (A–F) Representative images for each genotype and condition are shown in Figure 1—figure supplement 2. (G) qRT-PCR analyses measuring expression of NRE-GFP (left panel) and atilla (right panel). Transcript levels were quantified from hemolymph isolated from infested larvae at 0–6 hr or 24–30 hr post-infestation as indicated, relative to the untreated Su(H)gwt control. Tbp and cyp33 served as reference genes. Shown data were gained from four biological and two technical replicates each. Left panel: Immediately after wasp infection, NRE-GFP expression dropped significantly in the Su(H)gwt control, and even further to about 30% 24–30 hr post-infection, whereas it remained at 60–70% in the infested Su(H)S269A mutants. Right panel: atilla transcripts remained stable at first in the Su(H)gwt control, to rise dramatically 24–30 hr post-infection, in contrast to Su(H)S269A. Mini-max depicts 95% confidence, mean corresponds to expression ratio. Exact p-values are given in the raw data table. Significance was tested using PFRR from REST (*p<0.05).
-
Figure 1—source data 1
Raw data and statistical analysis.
- https://cdn.elifesciences.org/articles/89582/elife-89582-fig1-data1-v1.xlsx
Increased abundance of lamellocytes upon wasp infestation was monitored in vivo in larval hemolymph and lymph glands, using either the L1-atilla-GFP reporter (Honti et al., 2009) or PPO3-Gal4::UAS-GFP (Dudzic et al., 2015), respectively. In the harvested larval hemolymph of the infested Su(H)gwt control, the hemocytes contained about 15% PPO3- and 23% atilla-labelled lamellocytes (Figure 1C and D). These numbers were significantly lower in the wasp infested Su(H)S269A larvae (Figure 1C and D). Consistently, both lamellocyte reporters were robustly induced in the lymph glands of the infested control, but rarely in glands of the Su(H)S269A mutant (Figure 1E and F and Figure 1—figure supplement 2). Obviously, the Su(H)S269A mutant barely responds to the immune challenge raised by the parasitic wasp infestation.
In order to monitor the altered immune responses at the molecular level, we quantified Notch target gene expression in the hemolymph upon wasp infestation over time. We observed a decline in the expression of the Notch reporter NRE-GFP immediately after wasp infection in the control Su(H)gwt to about half the value of the uninfected larvae, dropping even further to about 30% 24–30 hr post-infection (Figure 1G). Su(H)S269A mutant larvae, however, retained a much stronger expression level at around 60–70% of the uninfected control even at the late time point. These data reveal the downregulation of Notch activity in response to wasp infestation prior or parallel to lamellocyte formation, in agreement with our model, whereby the infestation-induced phosphorylation of Su(H) impairs transmission of Notch signalling activity. Accordingly, the lamellocyte-specific marker atilla bounced up nearly two magnitudes in the wasp infected Su(H)gwt control at the late time point, but not in Su(H)S269A compared to the uninfected control (Figure 1G; Cattenoz et al., 2020).
Overall, these data support the model that S269 in Su(H) is a molecular target for a kinase, phosphorylated upon immune challenge to allow lamellocyte formation at the expense of crystal cells.
The phospho-deficient Su(H)S269A allele is impaired in combating wasp infestation
Parasitoid wasp infestation constitutes an extreme immune challenge for the Drosophila larva: if not combatted by the immune system, a wasp egg, which is deposited in the larval body cavity, will develop into an adult wasp, thereby killing the larval host during the pupal stage. Indeed, depending on the wasp species used, we measured a high mortality rate with less than 5% up to about 14% of surviving flies, whereas nearly all pupae hatched to adults without wasp challenge (Figure 2A). According to our working hypothesis, the phospho-deficient Su(H)S269A variant should not be able to properly respond to the immune challenge. To test this directly, we measured the survival of Su(H)S269A animals upon wasp infestation compared to the wild-type control.
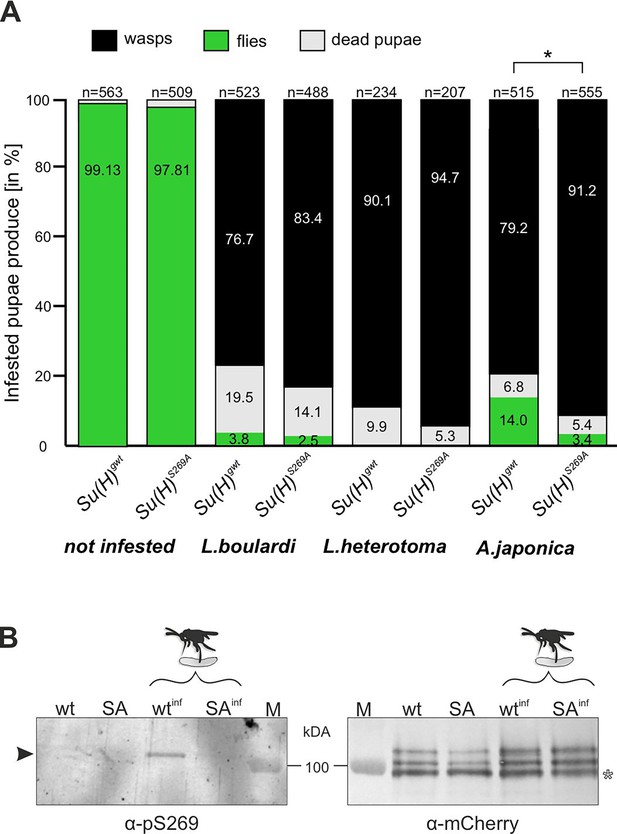
Resistance to wasp infestation and phosphorylation at S269.
(A) Resistance of Su(H)gwt and Su(H)S269A to the infestation with parasitic wasp strains L. boulardi and L. heterotoma (both family Figitidae) and Asobara japonica (family Braconidae), as indicated. Numbers of eclosed flies versus wasps as well as of dead pupae are presented in relation to the total of infested pupae. Left two columns are from non-infested controls. At least three independent experiments were performed, n=number of infested pupae. Statistically significant difference determined by Student’s t-test is indicated with *p<0.05. (B) Su(H) is phosphorylated at Serine 269 upon parasitoid wasp infestation. Protein extracts from Su(H)gwt-mCh (wt) and Su(H)S269A-mCh (SA) larvae, respectively, infested (wtinf, SAinf) with L. boulardi or uninfested, were isolated by RFP-Trap precipitation and probed in western blots. The anti-pS269 antiserum specifically detects wild-type Su(H) protein only in wasp infested larvae (arrowhead), but not the Su(H)S269A isoform. The blot on the right served as loading control, probed with anti-mCherry antibodies, revealing the typical Su(H) protein pattern in all lanes; the lowest band presumably stems from degradation (open asterisk). M, prestained protein ladder, protein size is given in kDa.
-
Figure 2—source data 1
Original, uncropped western blots shown in Figure 2B, probed with anti-pS269 and anti-mCherry, respectively.
- https://cdn.elifesciences.org/articles/89582/elife-89582-fig2-data1-v1.zip
-
Figure 2—source data 2
Original, uncropped western blots shown in Figure 2B, probed with anti-pS269 and anti-mCherry, respectively - with labelling.
Boxed areas correspond to regions shown in the main figure.
- https://cdn.elifesciences.org/articles/89582/elife-89582-fig2-data2-v1.zip
-
Figure 2—source data 3
Raw data and statistical analysis.
- https://cdn.elifesciences.org/articles/89582/elife-89582-fig2-data3-v1.xlsx
The two closely related wasp species L. boulardi and Leptopilina heterotoma (L. heterotoma) very efficiently parasitised both the control Su(H)gwt and the Su(H)S269A variant, though the latter appeared slightly more sensitive (Figure 2A). The difference in mortality became more apparent with the wasp species Asobara japonica (A. japonica), allowing 14% of the Su(H)gwt control flies to escape parasitism, whereas only 3.4% of the infested Su(H)S269A pupae emerged as flies. Thus, the Su(H)S269A mutants are less robust in resisting parasitoid wasp infestation consistent with an impaired immune response.
Serine 269 of Su(H) is phosphorylated upon wasp infestation
Next, we wanted to directly monitor Su(H) phosphorylation at S269 in response to wasp infestation. To this end, polyclonal antibodies directed against a phosphorylated peptide containing the sequence motif NRLRpSQTVSTRYLHVE were generated (⍺-pS269). Specificity was first tested by western blot analysis using bacterially expressed GST fusion proteins containing the entire beta-trefoil domain (BTD) of Su(H), as well as with phospho-mimetic (S269D) and phospho-mutant (S269A) versions. All three variants were detected by the antisera. The S269D version, however, was strongly preferred, indicating that this antibody does preferably recognise phospho-S269 Su(H) protein (Figure 2—figure supplement 1). Encouraged by this result, we used this antiserum on protein extracts derived from larvae infested and not infested by L. boulardi. For this experiment we used genome engineered fly strains expressing mCherry-tagged Su(H) proteins, Su(H)S269A-mCh and Su(H)gwt-mCh for control (Praxenthaler et al., 2017). Su(H) proteins were trapped using RFP-nanobody-coupled agarose beads, and the precipitates were then probed in western blots with antibodies directed against mCherry and pS269. Indeed, ⍺-pS269 antibodies recognised Su(H)gwt-mCh protein in wasp infested larvae, indicating respective phosphorylation of Su(H) protein. No such signals were seen in precipitates from the non-infected larvae nor from any of the Su(H)S269A-mCh mutant larvae (Figure 2B). These data strongly support the notion of parasitism-induced phosphorylation of Su(H) at S269. Apparently, parasitoid wasp infestation starts a cascade of events resulting in the inhibitory S269 phosphorylation of Su(H) protein to allow lamellocyte formation. Hence, the question arose on the kinase/s involved in this process.
Screening of kinase candidates mediating phosphorylation of Su(H) at Serine 269
To identify Ser/Thr kinases involved in the phosphorylation of Su(H) at S269, we commenced with a combination of in silico and biochemical approaches aiming to generate a list of candidate kinases which can be further analysed by genetic means (Figure 3). First, by using GPS 3.0 software that encompasses a substantial database of kinases and their preferred recognition motives (Xue et al., 2011), 36 potential human kinases were predicted to recognise S269 as substrate, represented by 30 kinases in Drosophila (Supplementary file 1). In addition, the BTD domain of Su(H) was bacterially expressed as a GST fusion protein and subjected to phospho-assays using 245 different human Ser/Thr kinases. Our reasoning for using the entire BTD domain was to ensure a normal folding of the domain (Kovall and Blacklow, 2010), and to reduce the number of potential phospho-sites at the same time present in full-length Su(H). With this approach, we ended up with 62 human Ser/Thr kinases (25% of the tested kinases) that use the BTD of Su(H) as an in vitro substrate, corresponding to 40 different kinases in Drosophila (Supplementary file 2). Both sets of data, biochemical and bioinformatics, were used to generate a list of 44 candidates to be analysed by genetic means. The candidates were further screened for an imbalanced hematopoiesis. We reasoned that mutants affecting a relevant kinase gene involved in the phosphorylation of Su(H) should display increased crystal cell numbers similar to what was observed in the Su(H)S269A mutant (Frankenreiter et al., 2021). Larvae of 13 different kinase mutants, and the progeny of 44 UAS-RNAi and/or UAS-kinase dead transgenes crossed with hml-Gal4, a blood cell-specific Gal4 driver line, were tested (Supplementary file 3). To this end, the larvae were subjected to heating for a visualisation and quantification of sessile crystal cells (Rizki, 1957; Lanot et al., 2001). About a third of the kinase mutants were similar to the control, whereas mutations in four kinases impeded crystal cell development or prevented it altogether. Unexpectedly, the majority of the tested kinase mutants exhibited elevated crystal cell numbers, however to a different degree (Figure 3—figure supplement 1, Supplementary file 4; Deichsel et al., 2024). Nineteen kinase mutants matched closely the Su(H)S269A phenotype, making those the most promising candidates to being involved in the phosphorylation of Su(H) at S269. Six of those were within the cluster of 10 candidates singled out by the in silico and the in vitro screens (Figure 3A and B). Using commercially available, activated human kinases, we were able to test five candidates, AKT1, CAMK2D, GSK3B, S6, and PKCα in vitro by MS/MS analysis on the Su(H) peptide ALFNRLRS8QTVSTRY, where Serine 8 (S8) corresponds to S269 in Su(H). Only PKCα unambiguously phosphorylated the given peptide at Serine 8. Whereas GSK3B did not phosphorylate the peptide at all, AKT1, CAMK2D, and S6 piloted Threonine 10, corresponding to Threonine 271 in Su(H) (Figure 3D, Figure 3—figure supplement 2). Together, these data support the idea that PKCα corresponding to Pkc53E in Drosophila is part of the kinase network mediating the phosphorylation of Su(H) at S269.
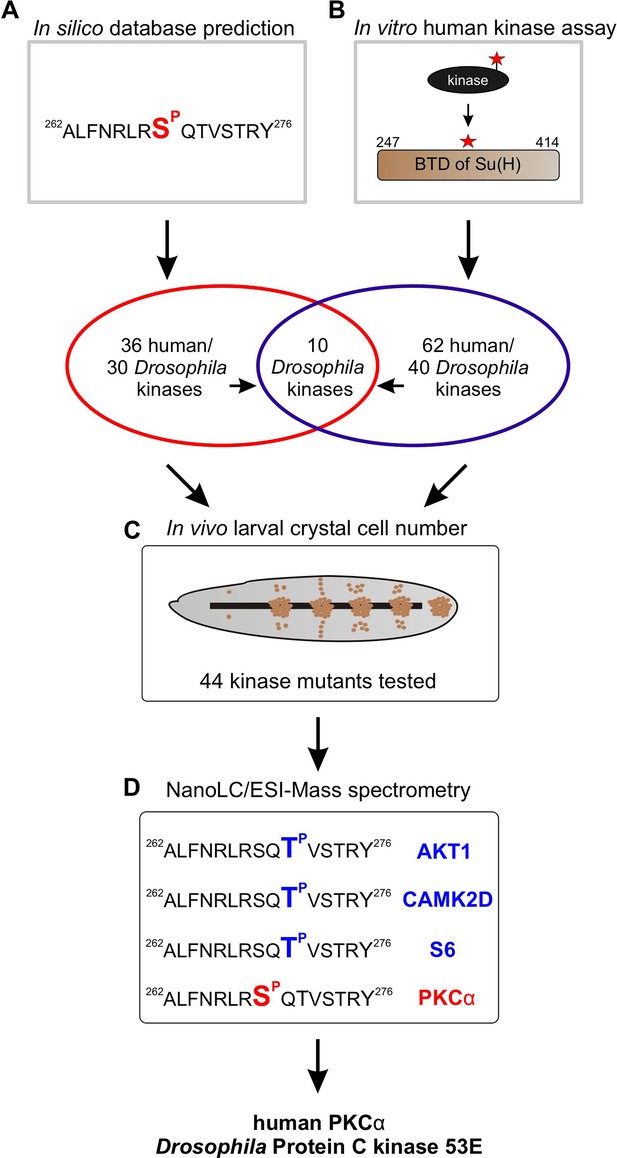
Pipeline of screening procedures for kinase candidates triggering phosphorylation at Su(H)S269.
(A) In silico screening of database(s) predicting kinase recognition motif in Su(H)S269; see Supplementary file 1. (B) In vitro assay screening 245 human Ser/Thr kinases for their ability to phosphorylate the beta-trefoil domain (BTD) domain of Su(H); see Supplementary file 2. (C) In vivo screen of 44 different Drosophila kinase mutants for crystal cell occurrence in third instar larvae; see Supplementary file 3, Supplementary file 4, and Figure 3—figure supplement 1. (D) NanoLC/ESI mass spectrometry with active human kinases monitoring their ability to phosphorylate the given Su(H) peptide. PKCα phosphorylates S269, whereas AKT1, CAMK2D, and S6 kinase prefer T271. Spectra are shown in Figure 3—figure supplement 2.
Role of Pkc53E in the phosphorylation of Su(H)S269
Confirming the MS/MS data, human PKCα was able to phosphorylate the respective Su(H) peptide (Swt) similar to its defined pseudosubstrate PS (Kochs et al., 1993), whereas the S8A mutant peptide (SSA) was accepted only half as well in an ADP-Glo assay, indicating that S269 is a preferred substrate (Figure 4A). Bacterially expressed and purified Drosophila Pkc53E, however, did neither accept the PS nor the Su(H) peptides (Figure 4B). Pkc53E activity, however, was stimulated by the agonistic phorbol ester PMA (phorbol 12-myristate 13-acetate) (Blumberg et al., 1983; Nakashima, 2002) to phosphorylate the PS and Su(H) peptide Swt but not the S8A mutant peptide SSA (Figure 4C). To generate an activated form of Pkc53E, we exchanged four codons by in vitro mutagenesis, three (T508D, T650D, and S669D) mimicking phosphorylation in the kinase and C-terminal domains, respectively, and one in the pseudosubstrate domain (A34E) (Figure 4—figure supplement 1; Gould and Newton, 2008). The resultant Pkc53EEDDD kinase accepted the PS, and the Su(H) peptide Swt even better, but not the S8A mutant peptide SSA, demonstrating the specificity of the phosphorylation (Figure 4D). As predicted for a fully activated kinase, PMA was unable to boost Pkc53EEDDD protein activity any further (Figure 4E). The bacterially expressed Pkc53EEDDD kinase, however, was a magnitude less active compared to commercial PKCα, perhaps reflecting poor quality of the bacterially expressed protein, or indeed an intrinsic biochemical property of the Drosophila enzyme.
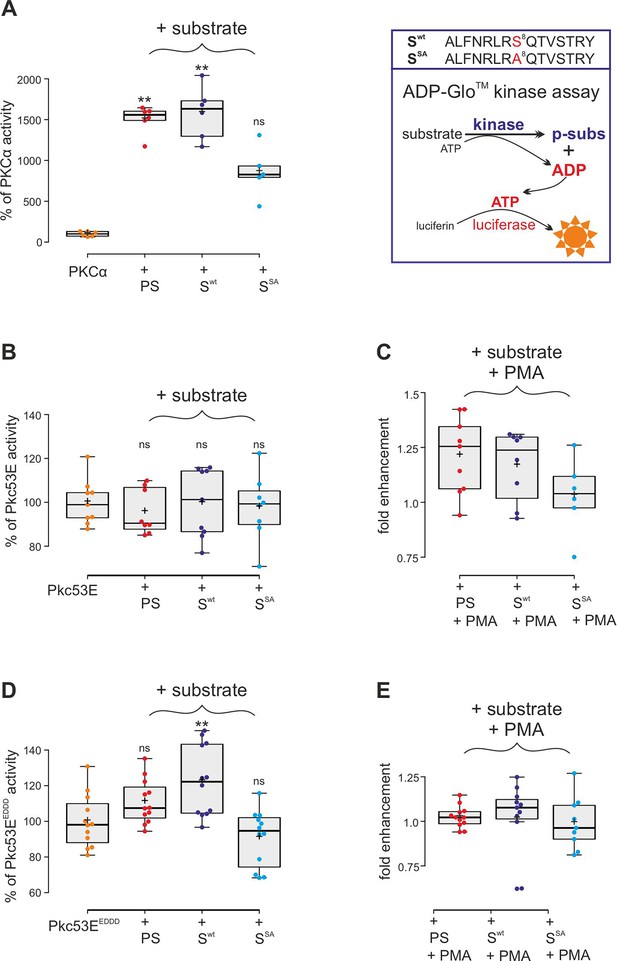
Kinase assays using activated PKCα and Drosophila Pkc53E variants.
(A) Right, schema of ADP-Glo assay to quantify kinase activity. The wild-type (Swt) and mutant (SSA) Su(H) peptides 262–276 offered as specific kinase substrates are indicated above. Left, the commercially available, active human PKCα very efficiently phosphorylates the pseudosubstrate PS and the Su(H) Swt peptide, but less efficiently the SSA mutant peptide. Activity is given as percentage of the auto-active kinase without substrate. Each dot represents one experiment (n=5-6). (B) Bacterially expressed Pkc53E has no activity on any of the offered substrates PS, Swt, or SSA (n=8-9). (C) PMA (phorbol 12-myristate 13-acetate) raised Pkc53E activity to nearly 125% for PS and Swt but not for SSA (n=6-9). (D) Activated Pkc53EDDD phosphorylates PS and Swt but not for SSA (n=12). (E) Addition of PMA does not change Pkc53EDDD activity (n=9-12). Statistical analyses were performed with Kruskal-Wallis test followed by Dunn’s test (A, C, E) or ANOVA followed by Tukey’s approach in (B, D) with **p<0.01, ns (not significant p≥0.05).
-
Figure 4—source data 1
Raw data and statistical analysis.
- https://cdn.elifesciences.org/articles/89582/elife-89582-fig4-data1-v1.xlsx
The PKC-agonist PMA influences Su(H) activity and blood cell homeostasis
Our data so far indicated that Su(H) is a phospho-target of Pkc53E which reduces its activity by affecting its DNA binding. In this case, we might expect an influence of the general PKC activator PMA on both, Su(H) activity and Notch-mediated crystal cell formation. We tested the former in a RBPJko HeLa cell system (Wolf et al., 2019), measuring Notch reporter gene activation by Su(H)-VP16. To this end, a Su(H)-VP16 gene fusion was cloned under HSV-TK promoter control, which is unresponsive to PMA in HeLa cells (Shifera and Hardin, 2009). Su(H)-VP16 protein is independent of Notch activity itself, allowing to directly monitor the influence of PMA on Su(H) activity. Indeed, Su(H)-VP16’s ability to activate reporter gene transcription was reduced by more than half in the presence of PMA (Figure 5A). This is in agreement with a PMA-mediated activation of endogenous PKC in the transfected HeLa cells, resulting in Su(H)-VP16 phosphorylation, loss of DNA-binding activity, and reduced transcriptional activation. Accordingly, repression could be reverted by the addition of kinase-inhibitor staurosporine (STAU) (Karaman et al., 2008). In fact, STAU alone already increased Su(H)-VP16 transcriptional activity, suggesting that inhibitory phosphorylation of Su(H) occurs in HeLa cells (Figure 5A). Expression levels of Su(H)-VP16, however, remained unchanged by the treatments (Figure 5—figure supplement 1).
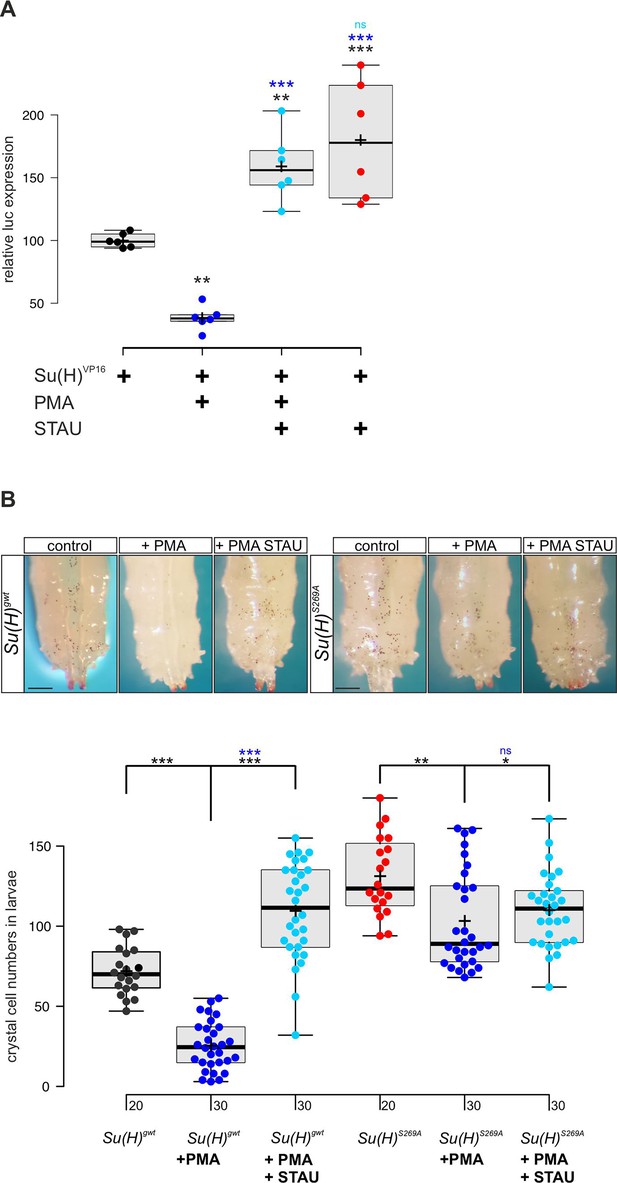
PMA (phorbol 12-myristate 13-acetate) inhibits Su(H) transcriptional activity in vitro and crystal cell formation in vivo.
(A) Expression of NRE-luciferase reporter gene in RBPjko HeLa cells, transfected with 2xMyc-Su(H)-VP16 [Su(H)VP16]. Luciferase activity is given relative to the reporter construct normalised to Su(H)-VP16 set to 100%. Addition of PMA causes reduction of Su(H)-VP16-dependent transcriptional activity to about 40%, which is reversed by the kinase inhibitor staurosporine (STAU). STAU itself results in increased Su(H)-VP16 activity. Each dot represents one experiment (n=6). Statistical analysis was performed with ANOVA followed by Dunnet’s multiple comparison test with ***p<0.001, **p<0.01 relative to Su(H)-VP16 alone (black asterisks) or to Su(H)-VP16 plus PMA (blue asterisks). (B) Number of melanised larval crystal cells determined in the last two segments of larvae fed with fly food plus 10% DMSO (control), or with fly food supplemented with 1 mM PMA, or 1 mM PMA plus 0.2 mM STAU (n=20 or n=30, as indicated). Note strong drop of crystal cell numbers in the Su(H)gwt control fed with PMA, and a reversal by STAU addition even above control levels. In contrast, PMA has a small effect on crystal cell number in the Su(H)S269A mutant, which is reversed by STAU. Representative animals are shown above; scale bar, 250 µm. Statistical analysis was performed by ANOVA followed by Tukey’s multiple comparison test (***p<0.001), significant differences are colour coded.
-
Figure 5—source data 1
Raw data and statistical analysis.
- https://cdn.elifesciences.org/articles/89582/elife-89582-fig5-data1-v1.xlsx
Next, we assayed the effect of PMA on larval crystal cell formation. If, as expected, PMA increased Pkc53E activity, Su(H) should be inactivated by phosphorylation with decreased crystal cell numbers as a consequence. We fed PMA to Drosophila larvae and assayed the numbers of sessile crystal cells. In agreement with our expectations, crystal cell numbers dropped very strongly, suggesting efficient phosphorylation and inactivation of Su(H) protein by PKCs (Figure 5B). As predicted by the above experiments, this effect was alleviated by STAU. Owing to the global inhibition of kinases by STAU, however, a rise in crystal cells was expected, because many kinases restrict their numbers (see Figure 3—figure supplement 1; Deichsel et al., 2024). In contrast, Su(H)S269A mutant larvae displayed an increased number of crystal cells which can be attributed to the fact that here, Su(H) can no longer be phosphorylated and hence, is overactive in this context. Feeding PMA to Su(H)S269A larvae caused only a minor drop of excessive crystal cell numbers, which could be due to other kinases acting on Su(H)S269 or due to other Pkc53E substrates involved in crystal cell development. Again, kinase inhibition by STAU largely reversed this effect (Figure 5B). In conclusion these data support the notion that Su(H) activity is regulated in vitro and in vivo by PKC activity in the context of blood cell homeostasis.
Pkc53E is required for normal blood cell homeostasis in Drosophila larvae
Su(H)S269A mutant larvae develop an excess of crystal cells, both in the hemolymph and in the lymph glands, due to a failure to downregulate respective Notch activity in the particular progenitor cells via the phosphorylation of Su(H) protein (Frankenreiter et al., 2021) (see Figure 1A and B). Assuming Pkc53E has a major role in the phosphorylation of Su(H), we should expect a similar phenotype in a Pkc53E mutant due to the inability to phosphorylate any substrate. In order to test this assumption directly, we assessed the number of crystal cells in larval lymph glands as well as in sessile crystal cells in several loss-of-function backgrounds of Pkc53E. To this end, we used the Pkc53EΔ28 allele, which by RT-PCR is a null mutant (Figure 6—figure supplement 1). In addition, we used two different RNAi-lines and one sgRNA line under UAS-control to knock down Pkc53E activity specifically in progenitor cells within the developing lymph gland using lz-Gal4 and in the hemolymph using hml-Gal4, respectively (Lebestky et al., 2000). As the hml-Gal4 driver is active in plasmatocytes and pre-crystal cells (Mukherjee et al., 2011; Tattikota et al., 2020), it should affect Pkc53E activity prior to crystal cell commitment in the hemolymph. However, within the lymph gland, hml appears specific to the plasmatocyte lineage and not present in crystal cell precursors. Instead, we choose lz-Gal4 for the Pkc53E knockdown, as lz is expressed in differentiating crystal cells of the lymph gland (Lebestky et al., 2000; Blanco-Obregon et al., 2020).
In any context tested, the number of crystal cells was strongly increased matching those of the Su(H)S269A mutant (Figure 6, Figure 6—figure supplement 2). The similar phenotypes imply that Pkc53E acts through the phosphorylation of Su(H) specifically within hemocytes to restrict crystal cell differentiation. However, as outlined above, the majority of kinase mutants displayed increased crystal cell numbers, raising the possibility of a fortuitous accordance. If the increase of crystal cell numbers in Pkc53E mutants is independent of Su(H) phosphorylation, we should expect an additive effect if we combine the two mutants. The double mutants Su(H)S269A Pkc53EΔ28 were generated by genetic recombination; they displayed the same range of excessive crystal cell numbers as the single mutants (Figure 7A and B, Figure 7—figure supplement 1). Moreover, the strongly reduced number of crystal cells observed in the Su(H)S269D mutant was not increased by Pkc53EΔ28 (Figure 7A and B), indicating that Pkc53E indeed acts upstream of Su(H), or directly on Su(H). If the latter is the case, we may expect the two proteins to form complexes in vivo. Indeed, we could co-precipitate Su(H)-Pkc53E protein complexes, both from Drosophila heads containing hemocytes (Sanchez Bosch et al., 2019), and from the larval hemolymph (Figure 7C and D). Specific co-precipitation was eased by using fly strains expressing m-Cherry-tagged Su(H) (Praxenthaler et al., 2017) and HA-tagged Pkc53E, respectively. Together, these data demonstrate that Pkc53E has an important role in blood cell homeostasis that can be largely explained by its activity to phosphorylate Su(H), thereby regulating the activity of Notch target genes during hemocyte and lymph gland development.
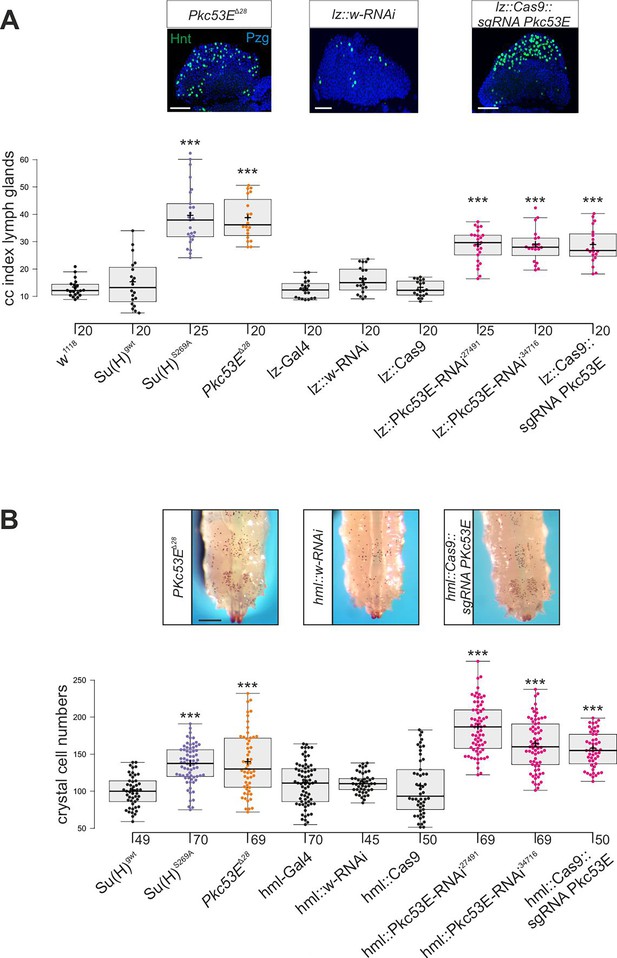
Loss of Pkc53E causes a gain of crystal cell number.
Depletion of Pkc53E activity in the Pkc53EΔ28 mutant or after knockdown by Pkc53E-RNAi or sgPkc53E with the help of the Gal4-UAS system using lz-Gal4 (A) or hml-Gal4 (B). Controls as indicated. (A) Crystal cell index in lymph glands; each dot represents the value of an analysed lobus (n=20 or n=25, as indicated). Examples of Pkc53EΔ28, lz::Pkc53-RNAi, and lz::Cas9 sgPkc53E are shown above. Crystal cells are labelled with Hnt (green), the lobe is stained with α-Pzg (blue). Scale bar, 50 µm. Representative images of lymph glands for each genotype are shown in Figure 6—figure supplement 2A. Statistical analysis by ANOVA followed by Tukey’s multiple comparison test relative to controls with ***p<0.001. (B) Melanised crystal cells enumerated from the last two segments of larvae with the given genotype (n=45–70 as indicated). Examples of respective Pkc53EΔ28, lz::Pkc53-RNAi, and lz::Cas9 sgPkc53E larvae are shown above. Scale bar, 250 µm. Representative larval images for each genotype are shown in Figure 6—figure supplement 2B. Statistical analysis by Kruskal-Wallis test, followed by Dunn’s test relative to controls with ***p<0.001. Note that there were no significant differences between any of the controls shown in black.
-
Figure 6—source data 1
Raw data and statistical analysis.
- https://cdn.elifesciences.org/articles/89582/elife-89582-fig6-data1-v1.xlsx
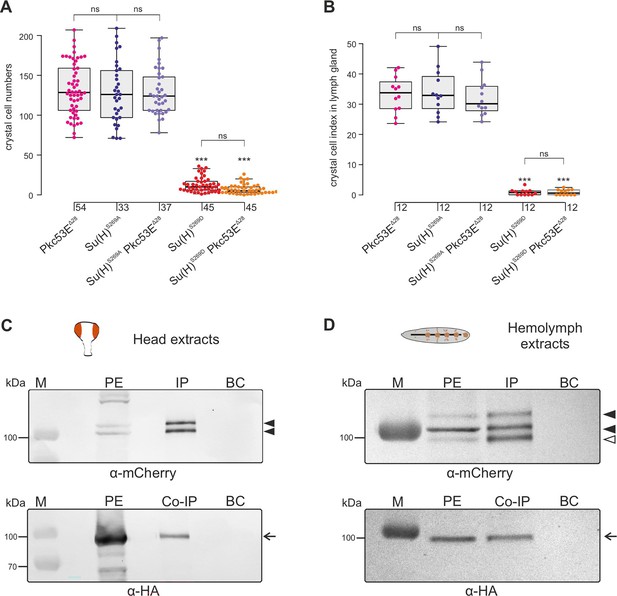
Pkc53E interacts with Su(H) at a genetic and a physiological level.
(A) Larval crystal cell numbers and (B) crystal cell indices in lymph glands were determined in the given genotypes. Each dot represents one analysed larva (n, as indicated) (A) or lymph gland lobus (n=12). (B) Statistical analysis by Kruskal-Wallis test, followed by Dunn’s test relative to controls with ***p<0.001; p≥0.05 ns (not significant). Representative images of sessile crystal cells and of lymph glands for each genotype are shown in Figure 7—figure supplement 1. (C, D) Co-immunoprecipitation of Pkc53EHA with Su(H)gwt-mCh protein. RFP-Trap IP was performed with protein extracts from 400 heads (C) or 25 third instar larvae (D), respectively. UAS-Pkc53E-HA expression was induced with Gmr-Gal4 in the head or with hml-Gal4 in the hemolymph. Endogenous mCherry-tagged Su(H) was trapped and detected with anti-mCherry antibodies (black arrowheads). The lowest band from the hemolymph is presumably a degradation product (open arrowhead in (D)). HA-tagged Pkc53E was specifically co-precipitated as detected with anti-HA antibodies (arrow). 10% of the protein extract (PE) used for the IP-Trap was loaded for comparison. BC corresponds to the Trap with only agarose beads as a control. M, prestained protein ladder; protein size is given in kDa.
-
Figure 7—source data 1
Original, uncropped western blots of Su(H)-mCh and Pkc53E-HA co-IP in head extracts and hemolymph, respectively, shown in Figure 7C and D.
- https://cdn.elifesciences.org/articles/89582/elife-89582-fig7-data1-v1.zip
-
Figure 7—source data 2
Original, uncropped western blots of Su(H)-mCh and Pkc53E-HA co-immunoprecipitation (co-IP) in head extracts and hemolymph, respectively, shown in Figure 7C and D - labelled.
Boxed areas correspond to the regions shown in the main figure.
- https://cdn.elifesciences.org/articles/89582/elife-89582-fig7-data2-v1.zip
-
Figure 7—source data 3
Raw data and statistical analysis.
- https://cdn.elifesciences.org/articles/89582/elife-89582-fig7-data3-v1.xlsx
Pkc53E mutants are immune-compromised
According to our hypothesis, Pkc53E phosphorylates Su(H) in response to an immune challenge by parasitic wasp infestation. In fact, a Pkc53E-eGFP fusion protein expressed from the Pkc53E locus via protein trap (Lee et al., 2018) was observed in the cytoplasm of all blood cell types independent of wasp infestation (Figure 8). Moreover, Pkc53E mRNA was expressed in cells of the larval hemolymph (Figure 8—figure supplement 1). Hence, we would expect that a loss of Pkc53E function should affect the ability of Drosophila larvae to fight wasp infestations similarly to the Su(H)S269A mutant. This was indeed the case. Firstly, the Pkc53EΔ28 null mutant was likewise impaired in fighting an infestation with the wasp A. japonica as was the Su(H)S269A mutant or the double mutant Su(H)S269A Pkc53EΔ28 with only about 4% surviving flies versus 14% in the control (Figure 9A). In contrast to Su(H)S269A, however, the Pkc53EΔ28 mutant larvae contained significantly lower hemocyte numbers independent of infestation, perhaps partly explaining the poor immune response (Figure 9—figure supplement 1; McGonigle et al., 2017). Without infestation, however, Pkc53EΔ28 mutant larvae developed normally to adulthood (Figure 9—figure supplement 1). Moreover, when Pkc53EΔ28 was infested with the parasitic wasp L. boulardi, lamellocyte numbers in the hemolymph did not reach wild-type levels, and they were almost absent from the larval lymph glands (Figure 9B and C). Apparently, the Pkc53EΔ28 null mutant is impaired in recognising parasitic wasp infestation or is hampered responding to it, e.g., by the phosphorylation of Su(H), demonstrating the involvement of this kinase in the immune response of Drosophila to parasitoid wasp infestation.
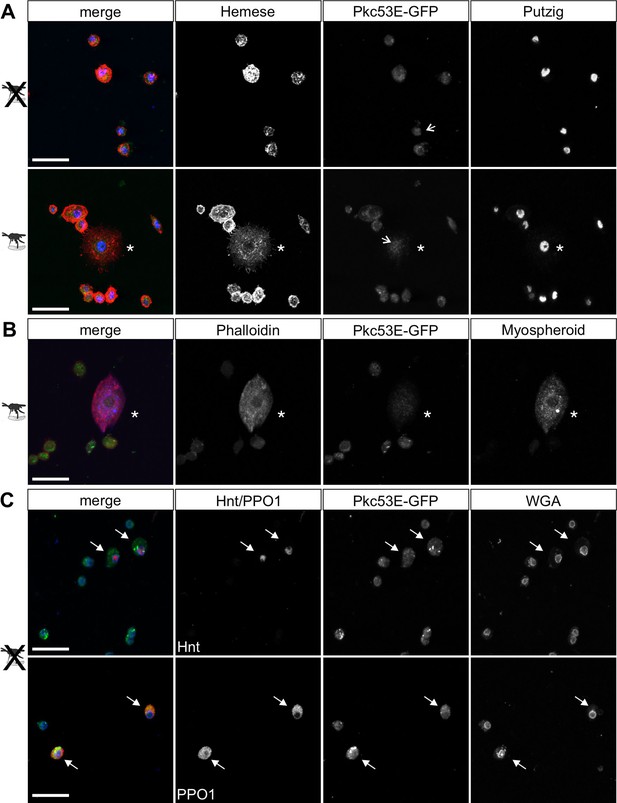
Pkc53E-eGFP is expressed in the cytoplasm of all hemocytes.
Hemocytes derived from Pkc53E-eGFP expressing larvae, either infested or not infested with L. boulardi were stained with the antibodies and compounds indicated. (A) Pkc53E-eGFP is present in the cytoplasm of hemocytes independent of wasp infection. Note complete overlap with Hemese (red) marking all types of blood cells; Putzig (blue) labels nuclei. Asterisk denotes lamellocyte in hemolymph of infected larvae. Arrows point to nuclei expressing Pkc53E-eGFP. (B) Pkc53E-eGFP is expressed in the cytoplasm of a lamellocyte (asterisk), labelled with myospheroid (blue) and rhodamine-coupled phalloidin (red). (C) Pkc53E-eGFP is enriched in the cytoplasm of crystal cells (arrow), labelled either with Hnt (red) or PPO1 (red), as indicated. Wheat germ agglutinin (WGA, blue) served to label nuclear lamina. Scale bar, 25 µm.
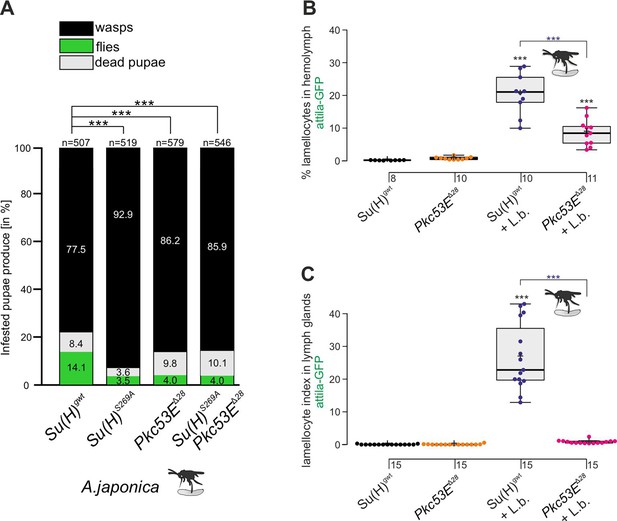
The Pkc53EΔ28 null mutant is immune-compromised.
(A) Resistance of Pkc53EΔ28 and the double mutant Su(H)S269A Pkc53EΔ28, compared to Su(H)gwt and Su(H)S269A for control, to the infestation with parasitic wasp strain A. japonica. Numbers of eclosed wasps versus flies as well as of dead pupae are presented in relation to the total of infested pupae (n, number of infested pupae). Statistical analysis by ANOVA followed by Tukey’s multiple comparison test relative to control Su(H)gwt; *p<0.05; ***p<0.001. (B, C) Quantification of lamellocytes labelled with the atilla-GFP reporter in the circulating hemolymph (B) or in the lymph glands (C), in uninfested conditions or upon wasp infestation as indicated. Representative images of hemolymph and of lymph glands for each genotype are shown in Figure 9—figure supplement 2. (B) Fraction of GFP-positive lamellocytes relative to the total of DAPI-stained hemocytes in the pooled hemolymph from 10 larvae. Each dot represents one larval pool (n, number of experiments as shown). (C) Lamellocyte index, i.e., number of GFP-labelled cells relative to the size of the lymph gland (n=15). Statistical analysis by ANOVA followed by Tukey’s multiple comparison test; ***p<0.001; significant differences are colour coded.
-
Figure 9—source data 1
Raw data and statistical analysis.
- https://cdn.elifesciences.org/articles/89582/elife-89582-fig9-data1-v1.xlsx
Discussion
The Notch pathway is highly conserved between invertebrates and vertebrates, with regard to both the underlying molecular principles and the biological processes it is involved, including hematopoiesis and immune defense. During mammalian hematopoiesis, Notch plays a fundamental role in stem cell maintenance and proliferation as well as in the differentiation of blood cell precursors, notably in T-cell development. Accordingly, aberrant Notch signalling activity has profound consequences for blood cell homeostasis that may result in leukemia (reviewed in Radtke et al., 2010; Siebel and Lendahl, 2017; Banerjee et al., 2019; Gallenstein et al., 2023). Hence, the principles of the regulation of Notch signalling activity are of great interest.
Notch signals are transduced by CSL-type DNA-binding proteins that are pivotal to Notch pathway activity, as no signal transduction could occur in their absence or in the instance of a lack of DNA binding. CSL proteins are extremely well conserved. They act as a molecular switch, either activating or repressing Notch target genes, depending on the recruited cofactors. Upon ligand binding, the Notch receptor is cleaved, and the biologically active Notch intracellular domain assembles an activator complex with CSL and further cofactors. In the absence of Notch receptor activation, however, CSL recruits co-repressors for gene silencing; in Drosophila repressor complex formation is mediated by Hairless (reviewed in Maier, 2006; Borggrefe and Oswald, 2009; Kovall and Blacklow, 2010; Bray, 2016; Giaimo et al., 2021). Accordingly, a loss of DNA binding by CSL is expected to affect both, the activation of Notch targets in the presence and their repression in the absence of Notch signals. This dual effect was observed in the context of wing development in cells homozygous for the DNA-binding defective Su(H)S269D variant: a failure of Notch target gene expression in areas of high Notch activity, as well as a de-repression of Notch target genes in areas outside (Frankenreiter et al., 2021). Phosphorylation of Su(H) hence entails not only the inhibition of Notch activity, but likewise a de-regulation of genes silenced by Su(H)-repressor complexes. Previously, we have shown that the regulation of Notch activity during hemocyte differentiation is independent of Hairless, but rather relies on the phosphorylation of Su(H) (Frankenreiter et al., 2021). Moreover, Notch activity needs to be downregulated before lamellocyte formation during parasitism, arguing for an inhibition of Notch activity rather than a de-repression of Notch target genes resulting from Su(H) phosphorylation during wasp parasitism.
Studies of Drosophila hematopoiesis have uncovered a pleiotropic role of Notch in all the hematopoietic compartments, where Notch activity needs to be precisely regulated to ensure blood cell homeostasis (reviewed in Banerjee et al., 2019; Csordás et al., 2021). During blood cell formation, Notch directs the crystal cell lineage. Accordingly, a downregulation of Notch activity causes a loss of crystal cells, whereas a gain of Notch activity results in increased numbers (Duvic et al., 2002; Lebestky et al., 2003; Terriente-Felix et al., 2013; Ghosh et al., 2015; Frankenreiter et al., 2021). In our earlier work, we have shown that the phosphorylation at S269 in the BTD of Su(H) impairs DNA-binding activity, and hence the capability of Su(H) to act as a transcriptional regulator in the context of blood cell development, without affecting Su(H) protein expression (Nagel et al., 2017; Frankenreiter et al., 2021). Accordingly, phospho-deficient Su(H)S269A mutants develop an excess of crystal cells. Now we provide evidence that Su(H) phosphorylation is likewise involved in parasitoid wasp defense, pointing to a dual use of the Notch pathway in blood cell homeostasis as well as in stress response, which is typical for the myeloid system (Banerjee et al., 2019).
The primary cellular immune response of Drosophila to fight parasitoid wasp infestation is an encapsulation of the parasite egg to terminate its further development. Albeit metabolically extremely costly, Drosophila larvae have to remodel their entire hematopoietic system to generate the masses of lamellocytes required for the encapsulation (reviewed in Letourneau et al., 2016; Kim-Jo et al., 2019; Csordás et al., 2021; Hultmark and Andó, 2022). There are two major sources for the lamellocytes. One is the transdifferentiation of circulating plasmatocytes, released from the sessile compartment and proliferating upon immune challenge (Márkus et al., 2009; Honti et al., 2010; Stofanko et al., 2010; Vanha-Aho et al., 2015; Anderl et al., 2016). Second is a massive expansion of prohemocytes in the lymph gland followed by a differentiation to lamellocytes and their release due to the premature disintegration of the gland (Lanot et al., 2001; Sorrentino et al., 2002; Louradour et al., 2017; Cho et al., 2020). Wasp infestation hence provokes a biased commitment to the lamellocyte lineage at the expense of crystal cells, as the two lineages are mutually exclusive (Krzemien et al., 2010; Tattikota et al., 2020; Cho et al., 2020; Cattenoz et al., 2020).
The processes underlying the Drosophila immune response to wasp parasitism are well understood, sharing many molecular details with the inflammatory responses of vertebrates (reviewed in Letourneau et al., 2016; Kim-Jo et al., 2019; Banerjee et al., 2019; Kharrat et al., 2022). Interestingly, sterile injury of Drosophila larvae initiates a likewise immune response covering all aspects of wasp-mediated immune challenge (Evans et al., 2022). The epidermal penetration by the wasp ovipositor causes a burst of hydrogen peroxide at the injury site via the activation of the NADPH oxidase DUOX. The oxidative stress then induces a systemic activation of Toll/NF-κB and JNK signalling within circulating hemocytes as well as within the cells of the posterior signalling centre of the lymph gland. Following cytokine release, JAK/STAT and EGFR signalling pathways are activated non-cell autonomously driving the (trans-)differentiation of (pro-)hemocytes to lamellocyte fate and lymph gland dispersal (Nappi et al., 1995; Schlenke et al., 2007; Sinenko et al., 2011; Gueguen et al., 2013; Razzell et al., 2013; Louradour et al., 2017; Chakrabarti and Visweswariah, 2020; Evans et al., 2022; reviewed in Letourneau et al., 2016; Banerjee et al., 2019). Whereas the switch to lamellocyte fate is well elaborated, less is known about the processes underlying the simultaneous suppression of crystal cell fate. Obviously, this step requires a downregulation of Notch signalling activity, as the Notch pathway is instrumental to crystal cell fate in all the hematopoietic compartments (Duvic et al., 2002; Lebestky et al., 2003; Schlenke et al., 2007; Small et al., 2014; reviewed in Banerjee et al., 2019). Our work now reveals that a parasitoid wasp attack causes a phosphorylation of Su(H) on S269 as means of an efficient and reversible way to inhibit Notch activity. This idea is consistent with earlier observations, whereby wasp parasitism quenched the expression of a Su(H)-lacZ reporter (Small et al., 2014). Moreover, we provide evidence that Pkc53E is involved in Su(H) phosphorylation during blood cell development as well as during parasitoid wasp defense in Drosophila. Moreover, the involvement of PKCs in wasp defense is not completely unexpected given their well-documented role in the immune responses of mammals and Drosophila alike. For example, Drosophila flies ensure a healthy gut-microbiota homeostasis by modulating DUOX activity as a pathogen-specific defense line (reviewed in Kim and Lee, 2014). DUOX activation and ROS production induced by gut pathogens was shown to be mediated by Pkc53E and Ca2+ via phospholipase Cβ (Ha et al., 2009; Lee et al., 2015). Sterile wounding of the Drosophila embryo triggers an instantaneous Ca2+ flash followed by hydrogen peroxide production (Razzell et al., 2013). It is conceivable that the epidermal breach by the wasp’s sting similarly induces a Ca2+ flash that may spark Pkc53E activation as a result. However, PKCs may be activated directly by oxidative modification even in the absence of Ca2+. Moreover, they may promote endogenous ROS production in a positive feedback loop (reviewed in Cosentino-Gomes et al., 2012), that could support the systemic response in the larval lymph gland.
The role of PKCs in the mammalian hematopoietic and immune systems is well documented. PKCs in this context act redundantly, and presumably, this holds also true for Drosophila where several PKCs exist. Upon activation, PKCs may translocate into the nuclear compartment, where they can directly influence gene expression programs, e.g., by piloting chromatin factors as substrates, pivotal for regulating immune cell differentiation (reviewed in Lim et al., 2015). Whereas Drosophila Pkc53E appears primarily cytoplasmic, its nuclear presence in some hemocytes is consistent with acting directly on Su(H) once activated (Figure 8A). Phosphorylation, however, may also occur at the membrane or in the cytoplasm, since Su(H) is imported into the nucleus together with its co-regulators (Wolf et al., 2019). PKCs, including PKCα, are present in CD34+ long-term hematopoietic stem cells. Moreover, specific roles in both the myeloid and the multilymphoid lineages are known. Notably, some PKC isoforms including PKCα may play a role in Notch-dependent T-cell development. Notch pathway activity is indispensable for T-cell lineage commitment of early thymic progenitors, however, is rapidly downregulated after the β-selection phase. As treatment with phorbol ester upregulates the transcription of TCRα and -β chains, a role of PKC in Notch-dependent commitment of αβ T-cells has been proposed (reviewed in Altman and Kong, 2016; Yui and Rothenberg, 2014). During initial T-cell development, Notch1 signalling increases in intensity, however, is abruptly downregulated at the transcriptional level following β-selection. This rapid downregulation is mediated by the inhibition of the E proteins (Yashiro-Ohtani et al., 2009), however, might in addition involve the phosphorylation of CSL by PKCα. Cell fate in more mature thymocytes is then fixed by the silencing of Notch target genes through chromatin modulators (reviewed in Yui and Rothenberg, 2014).
Appropriate silencing of Notch signalling activity in the course of T-cell development is of utmost importance, as prolonged Notch activity during β-selection predisposes the T-cells to leukemic transformation. Phosphorylation of CSL proteins by PKCα kinase offers a way for a timely and reversible deactivation of Notch signals not only in Drosophila but also in the mammalian system. In fact, the amino acid sequences harbouring the respective Serine residue in the CSL BTD are completely conserved between vertebrates and invertebrates (Wilson and Kovall, 2006; Nagel et al., 2017), raising the possibility of a likewise regulatory mechanism in mammalian hematopoiesis and immunity. Indeed, respective phosphorylation of the human CSL protein at the homologous position S195 was observed in human embryonic stem cells, where differentiation was induced by phorbol ester treatment (Rigbolt et al., 2011), consistent with an involvement of PKCs in this context as well. In conclusion, our work uncovers an important role for PKC-mediated downregulation of CSL activity, thereby ensuring blood cell homeostasis and an appropriate immune response to parasitoid wasp infestation in Drosophila. Future work may uncover, whether similar mechanisms apply to mammalian hematopoiesis and immunity as well.
Materials and methods
Key resources table, see Appendix 1.
Maintenance of parasitoid wasps and infection assay of D. melanogaster
L. boulardi, L. heterotoma, and A. japonica were kindly provided by B Häußling and J Stökl, Bayreuth, Germany (Weiss et al., 2015). Wasp species were co-cultured with wild-type Drosophila larvae at room temperature. To this end, about forty 3- to 5-day-old female wasps and twenty male wasps were co-incubated with second instar larvae for 5–7 days at room temperature. Every other day, fresh drops of honey water were added to the vial plug for feeding the wasps. After two weeks, all hatched flies were discarded. Wasps emerge about 30 days after the infestation.
For the infection assays, 50–100 staged Drosophila late second/early third instar larvae of the respective genotype were transferred onto apple juice plates with fresh yeast paste. 30 female and 20 male wasps aged between 3 and 6 days were added to the larvae, allowing to infect them for 4–6 hr. Afterwards, wasps were removed and larvae were allowed to develop further in vials with normal fly food. Wasps were only used once for each infection. Only infected larvae containing wasp egg were used for the subsequent experiments. After infestation, hemolymph or lymph glands were prepared at the time points indicated for the particular application, or the survival rate of wasps versus Drosophila imago was recorded.
Fly work and genetic analyses
Request a detailed protocolFly crosses were performed with 30–40 virgin females and 20 males to avoid overcrowding and stress. Combination/recombination of fly stocks was monitored by PCR genotyping using primers listed in Supplementary file 5. A complete list of the kinase mutant flies tested in the ‘larval kinase screen’ is found in Supplementary file 3. As reporter lines served atilla-GFP (BL23540) and PPO3-Gal4 UAS mCD8-GFP (named PPO3::GFP, Dudzic et al., 2015), hmlΔ-Gal4 UAS-GFP (named hml::GFP herein, BL30142) (Sinenko and Mathey-Prevot, 2004) and He-Gal4 UAS-GFP (BL8700) (Zettervall et al., 2004). For Gal4/UAS-based overexpression and RNAi-mediated knockdown, we used hml-Gal4 (BL30141), lz-Gal4 (Lebestky et al., 2000; obtained from M Crozatier, Université de Toulouse, France), UAS-μMCas9 (VDRC 340002), UAS-HA-Pkc53E (this study), UAS-white-RNAi (BL31231), UAS-Pkc53E-RNAi27491 (BL27491), UAS-Pkc53E-RNAi34716 (BL34716), UAS-sgRNA-Pkc53E (VDRC341127), and UAS-N-RNAi (BL7078). The strain vasa-φC31, 96E-attB/TM3 (Bischof et al., 2007) served for the generation of UAS-HA-Pkc53E flies. Su(H) controls and mutants comprised: Su(H)gwt, Su(H)gwt-mCh, Su(H)S269A, and Su(H)S269D/CyO-GFP (Praxenthaler et al., 2017; Frankenreiter et al., 2021). Su(H)S269A-mCh flies were produced in this study by a C-terminal in frame fusion of mCherry to the Su(H)S269A mutant gene followed by genomic integration of the construct via gene engineering as outlined before (Praxenthaler et al., 2017).
Generation of the UAS-HA-Pkc53E fly line
Request a detailed protocolPkc53E cDNA (DGRC GH03188) was PCR-amplified and subcloned via XhoI/XbaI in a modified pBT-HA vector, harbouring three copies of an HA-Tag generated via annealed oligos cloned into Acc65I/XhoI to generate pBT-3xHA-Pkc53E. HA-Pkc53E was then shuttled via Acc65I/XbaI in likewise opened pUAST-attB vector (Bischof et al., 2007). All cloning steps were sequence verified. Primers used for cloning are included in Supplementary file 5. Transgenic fly lines were then generated with the help of the φC31 integrase-based system using 96E as landing site (Bischof et al., 2007).
Analyses of Drosophila hematopoetic cells and tissues
Recording total hemocyte numbers
Request a detailed protocolHemocytes were visualised by GFP fluorescence. To this end, Su(H)gwt, Su(H)S269A, and Pkc53EΔ28 alleles, respectively, were combined with hml-Gal4 UAS-GFP (BL30142) and He-Gal4 UAS-GFP (BL8700) that together label the vast majority of hemocytes (Petraki et al., 2015). Larvae were vortexed at maximum speed with glass beads for 2 min, and the hemolymph was collected individually in 20 µl PBS. A fourth of the hemolymph was distributed in six Pap-pen wells of about 2 mm diameter. To ease staging and to avoid overcrowding, 4 hr egg collections were used, and larvae developed in batches of about 100 animals at 25°C until wandering third instar larval stage (ca. 120 hr after egg laying [AEL]). In case of wasp challenge, staged early L3 larvae were infested (ca. 90 hr AEL) and bled 20–32 hr thereafter to determine total hemocyte numbers. Only larvae containing wasp eggs were examined. GFP-positive hemocytes were visualised by epi-fluorescence microscopy on an Axioskop II (Zeiss, Jena), pictured with an EOS 700D camera (Canon, Japan). For counting cells, we followed earlier descriptions (Petraki et al., 2015). For reproducibility, the ‘trainable Weka segmentation’ plugin was used to demarcate cells from background (Eibe et al., 2016), followed by the ‘analyse particles’ plugin of ImageJ. Each Pap-pen well count was taken as a technical replicate for the individual larvae to determine the average hemocyte number per µl, expanded by 20 for the total hemocyte number per larva.
Determination of sessile larval crystal cells
Request a detailed protocolLarval crystal cells were counted according to Frankenreiter et al., 2021. Briefly, staged wandering third instar larvae of the respective genotype were heated to 60°C for 10–12 min. Pictures of the posterior dorsal side were taken with a Pixera camera (ES120, Optronics, Goleta, CA, USA) mounted to a stereo-microscope (Wild M3Z, Leica, Wetzlar, Germany) with Pixera Viewfinder 2.5. Melanised crystal cells appear as black dots, and were counted in the last two larval segments with ImageJ 1.51 software using Cell Counter tool. At least 20 larvae were scored for the statistical evaluation. For the PMA/STAU-feeding experiments, 20–30 developmentally synchronised second instar larvae were selected and grown for 24 hr in complete dark at 25°C on fly food with 200 µl of 1 mM PMA or PMA plus 0.2 mM STAU added to the surface of the fly food. As PMA and STAU were dissolved in DMSO before further dilution, controls were exposed accordingly to 10% DMSO on the fly food. Subsequently, wandering third instar larvae were heated and analysed as above.
Visualisation and quantification of lamellocytes
Request a detailed protocolThe lamellocyte specific reporters atilla-GFP or PPO3::GFP strains were re/combined with Su(H)gwt, Su(H)S269A, or Pkc53EΔ28 alleles by genetic means. Late second/early third larval instars were infested by L. boulardi and the number of lamellocytes was determined 2 days later and compared with those observed in non-infested larvae. Larvae were washed thoroughly in cold PBS and dried with a tissue and teared apart. The hemolymph of 10 larvae each was collected with a 20 μl Microloader tip (Eppendorf, Hamburg, Germany) and placed on a slide with 7 μl of Vectashield mounting medium containing DAPI. GFP-positive cells, i.e., lamellocytes were counted in relation to the total number of DAPI-labelled hemocytes with a Zeiss Axioskop II and a PlanNeofluar ×20 objective. 8–10 independent bleedings were performed each.
Pkc53E expression in hemocytes
Request a detailed protocolPkc53E-eGFP flies are derived from a protein trap and express endogenously a respective fusion protein (Lee et al., 2018). The hemolymph of three to five third instar Pkc53E-eGFP larvae at around 100 hr AEL, non-infested or infested at 80 hr AEL overnight with L. boulardi, was collected as described above in 20 μl cold PBS. Hemocytes were allowed to settle for 5–10 min in 500 µl cold PBS onto a round 18 mm glass slide placed in a 12-well microtiter plate. After fixation in 1 ml 4% paraformaldehyde in PBS for 15 min at room temperature, three washes with PBS plus 0.3% Triton X-100 (PBX), and a pre-incubation step in 500 µl 4% normal donkey serum in PBX for 45 min, cells were incubated overnight at 4°C with primary antibodies in 4% donkey serum in PBX (anti-GFP 1:100; anti-He 1:50; anti-Hnt 1:20, anti-mys 1:10, anti-PPO1 1:3; anti-Pzg 1:500), rhodamine-coupled phalloidin (1:200-1:400). Depending on the combination, staining with wheat germ agglutinin (WGA, 1:200) was performed for 15 min (hnt) and 60 min (PPO1) at room temperature. Three further washing steps and a pre-incubation step as above were followed by incubation for 2 hr at room temperature with suitable secondary antibodies (1:200) in the dark, followed by three additional washing steps. Cells were mounted in Vectashield by placing the round coverslip upside down on a glass slide, and pictures taken with a Bio-Rad MRC1024 coupled to Zeiss Axioskop with a PlanNeofluar ×63 objective using LaserSharp software 2000 (Zeiss, Jena, Germany).
Immunostaining and documentation of larval lymph glands
Request a detailed protocolLarval lymph glands were prepared 24–36 hr after wasp infection and treated as described before (Frankenreiter et al., 2021). For comparison, non-infested lymph glands were prepared. Primary antibodies used for staining: mouse anti-Hnt for crystal cells (1:20) and guinea pig anti-Pzg as nuclear marker (1:500). GFP signals were monitored directly. Secondary fluorescent antibodies were from Jackson ImmunoResearch Laboratories (1:250 each). Mounted tissue was documented with a Zeiss Axioskop coupled with a Bio-Rad MRC1024 confocal microscope using LaserSharp software 2000 (Zeiss, Jena, Germany). For statistical evaluation at least 12 primary lobes were documented and statistically analysed by using Image J software (Schindelin et al., 2012). Indices represent the number of cells in relation to the size/area of the tissue (in pixel) × 10,000.
RNA expression analyses
RT-PCR of Pkc53EΔ28 null mutants and in hemocytes
Request a detailed protocolPoly(A)+ RNA was isolated from 50 third instar larvae (Pkc53EΔ28 and y1w67c23) using PolyATract System Kit 1000 according to the manufacturer’s protocol, followed by a 10 min DNase I treatment at 37°C. Subsequent cDNA synthesis was conducted with qScriber cDNA Synthesis Kit according to the supplier’s protocol. For amplification, a Pkc53E primer pair overlapping the last three introns was chosen (Pkc53E_RT-PCR UP and Pkc53E_RT-PCR LP). Tubulin 56D primers (Tub56D_229 UP and Tub56D_507 LP) served as internal controls. For primers, see Supplementary file 5. In order to monitor Pkc53E expression in hemocytes, hemolymph was derived from 20 third instar Su(H)gwt larvae, and poly(A)+ RNA isolated using the Dynabeads micro mRNA-Kit, with an on-beads DNase I digest, otherwise following the above protocol with primer pair Pkc53E_RT-PCR UP and Pkc53E_RT-PCR LP.
Quantification of NRE-GFP transcription in hemocytes
Request a detailed protocolSu(H)gwt or Su(H)S269A stocks were genetically combined with NRE-GFP (BL30728). Hemolymph was collected from 15 to 30 early third instar larvae of each genotype, infested with L. boulardi for 6 hr at 72 hr AEL as described above, to be compared with non-infested Su(H)gwt control. Poly(A+) RNA was isolated directly thereafter (0–6 hr value) or 24 hr later (24–30 hr value) with Dynabeads mRNA (micro) Kit from the cells lysed in 200 µl lysis and binding buffer according to the manufacturer’s protocol, followed by an on-beads DNase I digest for 10 min at 37°C. After two washing steps, mRNA was eluted with 25 µl 10 mM Tris-HCl pH 8. cDNA synthesis was conducted with 15 µl using the qScriber cDNA Synthesis Kit according to the supplier’s protocol. Real-time qPCR was performed as described before using the Blue S’Green qPCR Kit and the MIC magnetic induction cycler (bms, Australia), including target and no-template controls (Kober et al., 2019). Four biological replicates with two technical replicates were each conducted. Results were compared to the ubiquitously expressed genes cyp33 and Tbp as reference. Primer pairs are listed in Supplementary file 5. The micPCR software version 2.12.7 was used for relative quantification of the data, based on REST and taking target efficiency into account (Pfaffl et al., 2002).
Determination of kinases and kinase assays
Screening of protein kinase candidates in silico and in vitro
Request a detailed protocolTo search for potential candidates in silico, GPS3.0 software was used at the lowest threshold levels, including the 40 kinases with the highest difference between score and cut-off value (Xue et al., 2011). The corresponding Drosophila kinases were determined with the help of flybase according to Morrison et al., 2000. For the in vitro screen, a 0.5 kb cDNA fragment (741–1242) encoding the Su(H) BTD (codons 247–414) was PCR-amplified and cloned via BamHI/EcoRI into pGEX-2T vector (Smith and Johnson, 1988) for bacterial expression and purification of the BTD-GST fusion protein. Primers used for cloning are included in Supplementary file 5. ProQinase GmbH (Freiburg, Germany) provided the ‘KinaseFinder assay service’. Briefly, BTD-GST and 33P-ATP served as substrates for 245 human Ser/Thr kinases in multi-well plates, analysed in a microplate scintillation reader. (A) Activity of each kinase was determined, (B) corrected for substrate background activity, and (C) auto-phosphorylation (kinase activity without substrate). A ratio value between phosphorylation of BTD-Su(H) and kinase auto-phosphorylation ≥1 (A-B/C) was considered as phosphorylating.
In vitro ADP-Glo kinase assay
Request a detailed protocolDrosophila pBT-3xHA-Pkc53E was mutated to generate the pseudo-activated form Pkc53EEDDD (A34E/T508D/T650D/S669D) stepwise by site-directed mutagenesis using the Q5 Site directed Mutagenesis Kit. Primers used for mutagenesis are included in Supplementary file 5. Pkc53E as well as PKC53EEDDD were then shuttled into a modified pMAL vector (Riggs, 1994), where additional restriction sites for Acc65I, SacII, and XhoI had been included in the multiple cloning site via primer annealing. The MBP-Pkc53E and MBP-Pkc53EEDDD fusion proteins were bacterially expressed and purified with Amylose resin. Additionally, activated human kinase PKCα was obtained as a positive control. The PKCα pseudosubstrate PS (RFARLGSLRQKNV) (Kochs et al., 1993), the wild-type Su(H) peptide Swt (ALFNRLRSQTVSTRY), and the phospho-deficient peptide SSA (ALFNRLRAQTVSTRY) were obtained (peptides & elephants, Hennigsdorf, Germany).
To test kinase activity, the ADP-Glo Kinase Assay system was used. Kinase assay reactions were performed in 96-well plates in a volume of 25 μl in the dark. Each reaction contained 100 μM of a kinase substrate peptide, 150 ng purified kinase, and 500 μM ultra-pure ATP. To stimulate kinase activity, 150 nM PMA was added to some reactions. The mixture was filled up with kinase reaction buffer (40 mM Tris-HCl, 20 mM MgCl2, 0.1 mg/ml BSA, pH 7.4) and incubated at room temperature for 1 hr in the dark. 25 μl ADP-Glo Reagent were added and incubated for 40 min to remove residual ATP. 50 μl of Kinase Detection Reagent was applied to convert ADP to ATP. The luminescent signal was measured after 45 min using GloMax Discover Microplate Reader (Promega, Madison, WI, USA), kindly provided by the Department of Zoology (190z), University of Hohenheim.
NanoLC-ESI-MS/MS analysis of Su(H) peptides
Request a detailed protocolNano-LC-ESI-MS/MS experiments were performed by the Mass Spectrometry Unit at the Core Facility Hohenheim (640) on an Ultimate 2000 RSLCnano system coupled to a Nanospray Flex Ion Source and a Q-Exactive HF-X mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Peptides were separated with LTQ-Orbitrap XL coupled to a nano-HPLC operated under the control of XCalibur 4.1.31.9 software (Thermo Fisher Scientific, Waltham, MA, USA). For all measurements using the Orbitrap detector, internal calibration was as described before (Olsen et al., 2005). MS/MS spectra were analysed using Proteome Discoverer 2.2 (Thermo Fisher Scientific, Waltham, MA, USA), verified by manual inspection of the MS/MS spectra (Voolstra et al., 2010).
Generation of a ⍺-pS269 antiserum
Request a detailed protocolRabbit polyclonal p-S269 Su(H) antiserum was generated by DAVIDS Biotechnology GmbH (Regensburg, Germany) using the synthetic phospho-peptide NRLRpSQTVSTRYLHVE. Phospho-specific antibodies were enriched in a depletion step by affinity purification against the non-phosphorylated peptide. We note a very low affinity of the purified antiserum for Su(H) protein. The antiserum detects purified Su(H)-GST fusion proteins (wild-type, S269A as well as S269D) with low affinity in western blots, but neither native Su(H) in tissue nor hemocytes nor in western blots, except if phosphorylated and enriched by Trap Technology.
Immunoprecipitation of mCherry and Myc-tagged Su(H)
Request a detailed protocol400 adult heads or 25 larvae of each genotype were homogenised on ice in 220 μl buffer 1 (150 mM NaCl, 1% Triton X-100, 50 mM Tris-HCl pH 7.5, 0.1% SDS, supplemented with protease inhibitor cocktail) and incubated for 15 min. After a short spin, 20 μl of the supernatant was set aside as input fraction (‘protein extract’). The residual supernatant was diluted with 300 μl wash buffer I (see buffer I, but without Triton X-100). 15 μl of equilibrated magnetic RFP-Trap Magnetic Agarose beads were added, incubated for 1 hr at 8°C and washed three times with wash buffer I. mCherry-trapped proteins were resolved on SDS-PAGE; western blots were probed with rabbit anti-mCherry (1:1000) and rat anti-HA (1:500). Goat secondary antibodies coupled with alkaline phosphatase (1:1000) were used for detection.
HeLa cell culture experiments and Luciferase assays
Generation of HSV-TK 2xMyc-Su(H)-VP16
Request a detailed protocolTwo myc-tags were added to Su(H) cDNA in pBT (Maier et al., 2011) by insertion of the two annealed oligonucleotides Myc-Tag UP and Myc-tag LP into the EcoRI site (for primers, see Supplementary file 5). The construct was subsequently shuttled via EcoRI/XhoI into pCDNA3.1. A VP16 activator domain was then cloned in frame at the C-terminus of 2xMyc-Su(H) by replacing the 829 bp BspEI/ApaI fragment of pCDNA3 2xMyc-Su(H) with a respective 1060 bp fragment of the pUAST Su(H)-VP16 construct (Cooper et al., 2000). Then the CMV Promoter of pCDNA3 2xMyc-Su(H)-VP16 was replaced by the HSV-TK Promoter of the pRL TK Vector. To this end, the 1023 bp BglII/NheI fragment from pRL TK was cloned into the BglII/SpeI opened pCDNA3 2xMyc-Su(H)VP16 construct.
Transfection of HeLa cells and reporter assay
Request a detailed protocolRBPjKO HeLa cells are RBPj-deficient HeLa cells (ATCC: CLL-2; DSMZ: Acc57; obtained from DSMZ), generated by CRISPR-Cas9 in the laboratories of F Oswald (University of Ulm) and T Borggrefe (University of Giessen), regularly monitored for mycoplasma contamination, confirmed by PCR and sequencing (Wolf et al., 2019). RBPjKO HeLa cells (#4.42) were cultivated and transfected as described (Wolf et al., 2019). The following constructs were used: pGL3 NRE-reporter (Bray et al., 2005), pCDNA3 HSV-TK 2xMyc Su(H)VP16, and pRL TK. For the Luciferase assay, 1×105 HeLa RBPjKO cells were seeded in each well of a 12-well cell culture plate. After 24 hr the cells were transfected with 500 ng pGL3 NRE-reporter, 460 ng pCDNA3 HSV-TK 2xMyc Su(H)VP16, and 40 ng pRL-TK. 4 hr after the transfection the cells were treated with 162 nM PMA, 162 nM PMA plus 21.4 nM STAU or 21.4 nM STAU alone. Control cells were treated with the same volume of DMSO present in the other treatments. 14 hr later, cells were washed twice in PBS pH 7.4 and lysed in 75 µl 1x Passive Lysis Buffer. The Dual-Luciferase Reporter Assay was performed according to the manufacturer’s instructions.
Su(H)-VP16 expression levels were detected with anti-Myc mAB (1:500) and anti-beta-tubulin as loading control (1:500). To this end, 5×105 HeLa RBPjKO cells were transfected and treated as above, harvested and lysed 14 hr later in 300 µl binding buffer (20 mM HEPES pH 7.6, 150 mM MgCl2, 10% glycerol, 0.05% NP-40, 1 mM DTT, ROCHE cOmplete ULTRA-tablet Mini protease inhibitor); 20 µl of each lysate were loaded for western blotting.
Statistical analysis and documentation of data
Request a detailed protocolNormality of the data was checked by a Shapiro-Wilk test using GraphPad Prism 9.0. In case of normally distributed data, a two-tailed analysis of variance (ANOVA) approach for multiple comparisons according to Tukey-Kramer’s Honestly Significance Difference or an unpaired t-test was applied, and in the other cases the non-parametric Kruskal-Wallis sum test or Dunn’s test for multiple comparisons. Quantification of RT-PCR data is based on REST, and was performed with the micPCR software version 2.12.7, taking target efficiency into account (Pfaffl et al., 2002). In the figures, p-values are presented as ***, p<0.001; **, p<0.01; *, p<0.05; not significant, p≥0.5; the exact p-values are given in the respective source data. Pictures were assembled using ImageJ, PhotoPaint, CorelDraw, and BoxPlotR software. In the box plots made by BoxPlotR, centre lines show the medians; box limits indicate the 25th and 75th percentiles as determined by R software; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles, outliers are represented by dots (Spitzer et al., 2014). Number of sample points is given in the figure or legend.
Appendix 1
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (D. melanogaster) | Pkc53E cDNA | Drosophila Genome Resource Center | GH03188 | |
| Recombinant DNA reagent | pBT-3xHA-Pkc53E | This paper | N/A, available upon request | HA-tagged Pkc53E subclone in pBT |
| Recombinant DNA reagent | Pkc53EEDDD | This paper | A34E/T508D/T650D/S669D | In vitro mutagenised 3xHA-Pkc53E cDNA |
| Gene (D. melanogaster) | Su(H) cDNA | Maier et al., 2011 | N/A | |
| Recombinant DNA reagent | Su(H) BTD in pGEX-2T | This paper | N/A, available upon request | For bacterial expression of a BTD-GST fusion protein |
| Recombinant DNA reagent | 2xMyc-Su(H) in pCDNA3.1 | This paper | N/A, available upon request | myc-tagged version of Su(H) |
| Recombinant DNA reagent | HSV-TK 2xMyc-Su(H)-VP16 | This paper | N/A, available upon request | myc-tagged version of Su(H) with VP16 activation domain under HSV control |
| Transfected construct (D. melanogaster) | HSV-TK 2xMyc-Su(H)-VP16 in RBPjKO HeLa cells | Wolf et al., 2019; this paper | N/A | Assay on the influence of PMA/Stau on Su(H)-VP16 activity |
| Recombinant DNA reagent | pGL3 NRE | Bray et al., 2005 | N/A | |
| Recombinant DNA reagent | pUAST Su(H)-VP16 | Cooper et al., 2000 | N/A | |
| Recombinant DNA reagent | pUAST-attB | Bischof et al., 2007 | RRID:DGRC_1419 | |
| Recombinant DNA reagent | pBT-3xHA | This paper | N/A, available upon request | Three HA-tags cloned into Acc65I/XbaI sites of pBT |
| Recombinant DNA reagent | pGEX-2T | Smith and Johnson, 1988 | N/A | |
| Recombinant DNA reagent | pMAL | Riggs, 1994 | N/A | |
| Recombinant DNA reagent | pCDNA3.1 | Invitrogen | Cat# V79020 | |
| Recombinant DNA reagent | pRL TK | Promega | Cat# E2241 | |
| Strain, strain background (Leptopilina boulardi) | Leptopilina boulardi | Häußling, J Stökl, Bayreuth | N/A | Parasitoid wasp |
| Strain, strain background (Leptopilina heterotoma) | Leptopilina heterotoma | Häußling, J Stökl, Bayreuth | N/A | Parasitoid wasp |
| Strain, strain background (Asobara japonica) | Asobara japonica | Häußling, J Stökl, Bayreuth | N/A | Parasitoid wasp |
| Genetic reagent (D. melanogaster) | atilla-GFP, i.e. w1118; Mi{ET1}atillaMB03539 | Bloomington Drosophila Stock Center | BDSC:23540 | |
| Genetic reagent (D. melanogaster) | He-Gal4 UAS-GFP, i.e. w*; P{He-GAL4.Z}85, P{UAS-GFP.nls}8 | Bloomington Drosophila Stock Center | BDSC:8700 | |
| Genetic reagent (D. melanogaster) | hml-Gal4, i.e. w1118; P{Hml-GAL4.Δ}3/MKRS | Bloomington Drosophila Stock Center | BDSC:30141 | |
| Genetic reagent (D. melanogaster) | hmlΔ-Gal4 UAS-GFP, i.e. w1118; P{Hml-GAL4.Δ}3, P{UAS-2xEGFP}AH3/MKRS | Bloomington Drosophila Stock Center | BDSC:30142 | |
| Genetic reagent (D. melanogaster) | PPO3-Gal4 UAS mCD8-GFP | Dudzic et al., 2015 | N/A | |
| Genetic reagent (D. melanogaster) | lz-Gal4 | Lebestky et al., 2000 | N/A | |
| Genetic reagent (D. melanogaster) | NRE-GFP, i.e. w1118; P{NRE-EGFP.S}1 | Bloomington Drosophila Stock Center | BDSC:30728 | |
| Genetic reagent (D. melanogaster) | Pkc53E-EGFP, i.e. Pkc53EMI05296-GFSTF.0 | Bloomington Drosophila Stock Center | BDSC:59413 | |
| Genetic reagent (D. melanogaster) | UAS-white-RNAi, i.e. y1 v1; P{TRiP.JF01574}attP2/TM3, Ser1 | Bloomington Drosophila Stock Center | BDSC:31231 | |
| Genetic reagent (D. melanogaster) | UAS-N-RNAi, i.e. P{UAS-N.RNAi.P}14E, w* | Bloomington Drosophila Stock Center | BDSC:7078 | |
| Genetic reagent (D. melanogaster) | UAS-sgRNA-Pkc53E | Vienna Drosophila Resource Center | VDRC341127 | |
| Genetic reagent (D. melanogaster) | UAS-µMCas9 | Vienna Drosophila Resource Center | VDRC 340002 | |
| Genetic reagent (D. melanogaster) | UAS-HA-Pkc53E | This study | N/A | HA-Pkc53E under UAS-control integrated at 96E (3R) |
| Genetic reagent (D. melanogaster) | vasa-φC31; 96E-attB/TM3 | Bischof et al., 2007 | N/A | |
| Genetic reagent (D. melanogaster) | Su(H)gwt | Praxenthaler et al., 2017 | N/A | |
| Genetic reagent (D. melanogaster) | Su(H)gwt-mCh, i.e. y1 w*; TI{TI}Su(H)gwt-mCh | Praxenthaler et al., 2017 | BDSC:94607 | |
| Genetic reagent (D. melanogaster) | Su(H)S269A, i.e. y1 w*; TI{TI}Su(H)S269A | Frankenreiter et al., 2021 | BDSC:94609 | |
| Genetic reagent (D. melanogaster) | Su(H)S269D/CyO-GFP, i.e. y1 w*; TI{TI}Su(H)S269D/CyO, P{GAL4-Hsp70.PB}TR1, P{UAS-GFP.Y}TR1 | Frankenreiter et al., 2021 | BDSC:94610 | |
| Genetic reagent (D. melanogaster) | Su(H)S269A-mCh, i.e. y1 w*; TI{TI}Su(H)S269A-mCh | This study | N/A | Knock-in allele of mCherry-tagged Su(H)S269A into the native Su(H) locus |
| Genetic reagent (D. melanogaster) | Kinase mutant flies tested in the larval kinase screen are listed in Supplementary file 3 | BDSC, VDRC, and various donors as indicated in Supplementary file 3 | ||
| Cell line (H. sapiens) | RBPjKO HeLa cells (origin is ATCC: CLL-2; DSMZ: ACC57) | Wolf et al., 2019; gift of F Oswald (University of Ulm) and T Borggrefe (University of Giessen) | N/A | Homozygous knockout of the RBPj gene |
| Antibody | Mouse monoclonal anti-Hnt, 1G9 | Developmental Studies Hybridoma Bank, developed by H Lipshitz | RRID: AB_528278 | IF(1:20) |
| Antibody | Mouse monoclonal anti-mys, CF.6G11 | Developmental Studies Hybridoma Bank, developed by D Brower | RRID: AB_528310 | IF(1:10) |
| Antibody | Mouse monoclonal anti-beta tubulin, E7 | Developmental Studies Hybridoma Bank, developed by M Klymkowsky | RRID: AB_2315513 | WB(1:500) |
| Antibody | Mouse monoclonal anti-myc 9B11 | Cell Signaling Techn. | RRID: AB_331783; Cat# 2276 | WB(1:500) |
| Antibody | Mouse monoclonal anti-Hemese | Kurucz et al., 2003; gift from I Andó, Szeged, Hungary | N/A | IF(1:50) |
| Antibody | Guinea pig polyclonal anti-Pzg | Kugler and Nagel, 2007 | N/A | IF(1:500) |
| Antibody | Mouse monoclonal anti-PPO1, 12F6 | Trenczek and Bennich, 1992; gift from TE Trenczek, Giessen, Germany | N/A | IF(1:3) |
| Antibody | Mouse monoclonal anti-GST (8-326) | Invitrogen | S RRID: AB_10979611, Cat# MA4-004 | WB(1:1000) |
| Antibody | Rabbit polyclonal anti-pS269 | This paper, DAVIDS Biotechnology GmbH | N/A | WB(1:100) |
| Antibody | Rabbit polyclonal anti-GFP | Santa Cruz | RRID: AB_641123; Cat# sc-8334 | IF(1:100) |
| Antibody | Rabbit polyclonal anti-mCherry | GeneTex | RRID: AB_2721247; Cat# GTX128508 | WB(1:1000) |
| Antibody | Rat monoclonal anti-HA 3F10 | ROCHE | RRID:AB_390918 Cat# 11867423001 | WB(1:500) |
| Antibody | Donkey polyclonal anti-mouse IgG, Cy3 | Jackson ImmunoResearch Laboratories | RRID: AB_2315777 Cat# 715-165-151 | IF(1:200) |
| Antibody | Donkey polyclonal anti-mouse IgG, Cy5 | Jackson ImmunoResearch Laboratories | RRID: AB_2340820 Cat# 715-175-151 | IF(1:200) |
| Antibody | Donkey polyclonal anti- guinea pig IgG, Cy5 | Jackson ImmunoResearch Laboratories | RRID: AB_2340462 Cat# 706-175-148 | IF(1:200) |
| Antibody | Donkey polyclonal anti- rabbit IgG, FITC | Jackson ImmunoResearch Laboratories | RRID: AB_2315776 Cat# 711-095-152 | IF(1:200) |
| Antibody | Goat polyclonal anti-guinea pig IgG, Alexa Fluor 647 | Jackson ImmunoResearch Laboratories | RRID: AB_2337446; Cat# 106-605-003 | IF(1:200) |
| Antibody | Goat polyclonal anti-mouse IgG, FITC | Jackson ImmunoResearch Laboratories | RRID: AB_2338601; Cat# 1115-095-166 | IF(1:200) |
| Antibody | Goat polyclonal anti-rabbit IgG, alkaline phosphatase | Jackson ImmunoResearch Laboratories | RRID: AB_2337947; Cat# 111-055-003 | WB(1:1000) |
| Antibody | Goat polyclonal anti-rat IgG, alkaline phosphatase | Jackson ImmunoResearch Laboratories | RRID: AB_2338148; Cat# 112-055-003 | WB(1:1000) |
| Antibody | Goat polyclonal anti-mouse IgG, alkaline phosphatase | Jackson ImmunoResearch Laboratories | RRID: AB_2338528; Cat# 115-055-003 | WB(1:1000) |
| Other | Normal donkey serum | Jackson ImmunoResearch Laboratories | RRID: AB_2337258 Cat# 017-000-121 | IF(1:400) |
| Other | Normal goat serum | Jackson ImmunoResearch Laboratories | RRID: AB_2336990 Cat# 005-000-121 | IF(1:400) |
| Peptide, recombinant protein | Activated PKCα | ProQinase | Cat# 0222-0000-1 | |
| Peptide, recombinant protein | Activated Akt1 | ProQinase | Cat# 1379-0000-2 | |
| Peptide, recombinant protein | Activated GSK3 beta | ProQinase | Cat# 0310-0000-1 | |
| Peptide, recombinant protein | Activated S6K | ProQinase | Cat# 0318-0000-2 | |
| Peptide, recombinant protein | CAMK2D | Invitrogen | NP_742113 | |
| Peptide, recombinant protein | Pseudosubstrate | peptides & elephants | PS | RFARLGSLRQKNV |
| Peptide, recombinant protein | Su(H) peptide | peptides & elephants | Swt | ALFNRLRSQTVSTRY |
| Peptide, recombinant protein | Su(H)SA peptide | peptides & elephants | SSA | ALFNRLRAQTVSTRY |
| Peptide, recombinant protein | Su(H) phosphopeptide | DAVIDS Biotechnology GmbH | N/A | NLRLpSQTVSTRYLHVE |
| Commercial assay or kit | RFP-Trap Magnetic Agarose | ChromoTek | Cat# rtma-20 | |
| Commercial assay or kit | Amylose resin | New England Biolabs GmbH | Cat# E8021S | |
| Commercial assay or kit | ADP-Glo Kinase Assay | Promega | Cat# V6930 | |
| Commercial assay or kit | PolyATract System Kit 1000 | Promega | Cat# Z5400 | |
| Commercial assay or kit | Dynabeads mRNA DIRECT micro purification kit | Invitrogen, Thermo Fisher | Cat# 61021 | |
| Commercial assay or kit | qScriber cDNA Synthesis Kit | highQu | Cat# RTK0104 | |
| Commercial assay or kit | Q5 Site directed Mutagenesis Kit | New England Biolabs GmbH | Cat# E0554S | |
| Commercial assay or kit | Blue S’Green qPCR Kit | Biozym | Cat# 331416 | |
| Commercial assay or kit | Dual-Luciferase Reporter Assay | Promega | Cat# E1910 | |
| Commercial assay or kit | Pap-pen | Kisker Biotech | Cat# MKP-1 | |
| Chemical compound, drug | DAPI | Cell Signaling Techn. | Cat# 4083 | (1 µg/ml) |
| Chemical compound, drug | DNase I | New England Biolabs GmbH | Cat# M0303 | (2 U/µl) |
| Chemical compound, drug | PMA, Phorbol-12-myristat-13-acetat | Sigma-Aldrich | Cat# P8139-1MG | (1 mM) |
| Chemical compound, drug | Staurosporine | Sigma-Aldrich | Cat# S4400-1MG | (0.2 mM) |
| Chemical compound, drug | Protease inhibitors, cOmplete ULTRA-tablets Mini | Roche | Cat# 5892791001 | (1 tablet/10 ml) |
| Chemical compound, drug | PhosSTOP (Phosphatase inhibitor) | Roche | Cat# 4906837001 | (1 tablet/10 ml) |
| Chemical compound, drug | Wheat germ agglutinin (WGA), Alexa Fluor 647 conjugate | Fisher Scientific | Cat# 11510826 | (1:200) |
| Chemical compound, drug | Phalloidin, coupled to rhodamine | Invitrogen, Thermo Fisher | Cat# R415 | (1:200-1:400) |
| Chemical compound, drug | Vectashield | Biozol | Cat# VEC-H_1000 | Mounting medium |
| Software, algorithm | GPS3.0 software | Xue et al., 2011 | ||
| Software, algorithm | ImageJ 1.51 | Schindelin et al., 2012 | https://imagej.nih.gov/ij/ | |
| Software, algorithm | GraphPad Prism version 9.0 | GraphPad Software, Inc | https://www.graphpad.com/ | |
| Software, algorithm | MIC PCR software version v2.12.7 | bms/Biozym | Cat# 68MiC-HRM | |
| Software, algorithm | Weka machine learning and data analysis software version 3.8 | Eibe et al., 2016 | https://waikato.github.io/weka-site/index.html | |
| Sequence-based reagent | Supplementary file 5, oligonucleotides | Microsynth AG |
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files; source data files have been provided for all figures.
References
-
Protein kinase C enzymes in the hematopoietic and immune systemsAnnual Review of Immunology 34:511–538.https://doi.org/10.1146/annurev-immunol-041015-055347
-
Context-specific functions of notch in Drosophila blood cell progenitorsDevelopmental Biology 462:101–115.https://doi.org/10.1016/j.ydbio.2020.03.018
-
Phorbol ester receptors and the in vitro effects of tumor promotersAnnals of the New York Academy of Sciences 407:303–315.https://doi.org/10.1111/j.1749-6632.1983.tb47836.x
-
The Notch signaling pathway: transcriptional regulation at notch target genesCellular and Molecular Life Sciences 66:1631–1646.https://doi.org/10.1007/s00018-009-8668-7
-
Bre1 is required for notch signaling and histone modificationDevelopmental Cell 8:279–286.https://doi.org/10.1016/j.devcel.2004.11.020
-
Notch signalling in contextNature Reviews. Molecular Cell Biology 17:722–735.https://doi.org/10.1038/nrm.2016.94
-
Temporal specificity and heterogeneity of Drosophila immune cellsThe EMBO Journal 39:e104486.https://doi.org/10.15252/embj.2020104486
-
The transcriptional diversity of 25 Drosophila cell linesGenome Research 21:301–314.https://doi.org/10.1101/gr.112961.110
-
Spatially restricted factors cooperate with notch in the regulation of enhancer of split genesDevelopmental Biology 221:390–403.https://doi.org/10.1006/dbio.2000.9691
-
Cell signaling through protein kinase C oxidation and activationInternational Journal of Molecular Sciences 13:10697–10721.https://doi.org/10.3390/ijms130910697
-
BookThe WEKA Workbench. Online Appendix for ‘Data Mining: Practical Machine Learning Tools and TechniquesMorgan Kaufmann, Fourth Edition.
-
Phospho-site mutations in transcription factor suppressor of hairless impact notch signaling activity during hematopoiesis in DrosophilaFrontiers in Cell and Developmental Biology 9:e658820.https://doi.org/10.3389/fcell.2021.658820
-
Notch signaling in acute inflammation and sepsisInternational Journal of Molecular Sciences 24:3458.https://doi.org/10.3390/ijms24043458
-
Transcription factor RBPJ as a molecular switch in regulating the notch responseAdvances in Experimental Medicine and Biology 1287:9–30.https://doi.org/10.1007/978-3-030-55031-8_2
-
The life and death of protein kinase CCurrent Drug Targets 9:614–625.https://doi.org/10.2174/138945008785132411
-
In vivo detection of lamellocytes in Drosophila melanogasterImmunology Letters 126:83–84.https://doi.org/10.1016/j.imlet.2009.08.004
-
A quantitative analysis of kinase inhibitor selectivityNature Biotechnology 26:127–132.https://doi.org/10.1038/nbt1358
-
Peeling back the layers of lymph gland structure and regulationInternational Journal of Molecular Sciences 23:7767.https://doi.org/10.3390/ijms23147767
-
Role of DUOX in gut inflammation: lessons from Drosophila model of gut-microbiota interactionsFrontiers in Cellular and Infection Microbiology 3:116.https://doi.org/10.3389/fcimb.2013.00116
-
Activation and substrate specificity of the human protein kinase C alpha and zeta isoenzymesEuropean Journal of Biochemistry 216:597–606.https://doi.org/10.1111/j.1432-1033.1993.tb18179.x
-
Mechanistic insights into notch receptor signaling from structural and biochemical studiesCurrent Topics in Developmental Biology 92:31–71.https://doi.org/10.1016/S0070-2153(10)92002-4
-
Hematopoietic progenitors and hemocyte lineages in the Drosophila lymph glandDevelopmental Biology 346:310–319.https://doi.org/10.1016/j.ydbio.2010.08.003
-
putzig is required for cell proliferation and regulates notch activity in DrosophilaMolecular Biology of the Cell 18:3733–3740.https://doi.org/10.1091/mbc.e07-03-0263
-
Postembryonic hematopoiesis in DrosophilaDevelopmental Biology 230:243–257.https://doi.org/10.1006/dbio.2000.0123
-
A serrate-expressing signaling center controls Drosophila hematopoiesisGenes & Development 17:348–353.https://doi.org/10.1101/gad.1052803
-
Structural and functional analysis of the repressor complex in the Notch signaling pathway of Drosophila melanogasterMolecular Biology of the Cell 22:3242–3252.https://doi.org/10.1091/mbc.E11-05-0420
-
Protein kinases and phosphatases in the Drosophila genomeThe Journal of Cell Biology 150:F57–F62.https://doi.org/10.1083/jcb.150.2.f57
-
Protein kinase C alpha (PKC alpha): regulation and biological functionJournal of Biochemistry 132:669–675.https://doi.org/10.1093/oxfordjournals.jbchem.a003272
-
Superoxide anion generation in Drosophila during melanotic encapsulation of parasitesEuropean Journal of Cell Biology 68:450–456.
-
Parts per million mass accuracy on an orbitrap mass spectrometer via lock mass injection into a C-trapMolecular & Cellular Proteomics 4:2010–2021.https://doi.org/10.1074/mcp.T500030-MCP200
-
Assaying blood cell populations of the Drosophila melanogaster larvaJournal of Visualized Experiments 1:52733.https://doi.org/10.3791/52733
-
Expression and purification of maltose‐binding protein fusionsCurrent Protocols in Molecular Biology 28:16.https://doi.org/10.1002/0471142727.mb1606s28
-
Alterations in the haemocyte population of Drosophila melanogasterJournal of Morphology 100:437–458.https://doi.org/10.1002/jmor.1051000303
-
Fiji: an open-source platform for biological-image analysisNature Methods 9:676–682.https://doi.org/10.1038/nmeth.2019
-
Cell lines derived from late embryonic stages of Drosophila melanogasterJournal of Embryology and Experimental Morphology 27:353–365.https://doi.org/10.1242/dev.27.2.353
-
PMA induces expression from the herpes simplex virus thymidine kinase promoter via the activation of JNK and ERK in the presence of adenoviral E1A proteinsArchives of Biochemistry and Biophysics 490:145–157.https://doi.org/10.1016/j.abb.2009.08.013
-
Notch signaling in development, tissue homeostasis, and diseasePhysiological Reviews 97:1235–1294.https://doi.org/10.1152/physrev.00005.2017
-
BoxPlotR: a web tool for generation of box plotsNature Methods 11:121–122.https://doi.org/10.1038/nmeth.2811
-
Characterization of haemocytes of Hyalophora cecropia by monoclonal antibodiesVerhandlungen Der Deutschen Zoologischen Gesellschaft 85:e216.
-
Light-dependent phosphorylation of the Drosophila transient receptor potential ion channelThe Journal of Biological Chemistry 285:14275–14284.https://doi.org/10.1074/jbc.M110.102053
-
Nucleo-cytoplasmic shuttling of Drosophila Hairless/Su(H) heterodimer as a means of regulating Notch dependent transcriptionBiochimica et Biophysica Acta. Molecular Cell Research 1866:1520–1532.https://doi.org/10.1016/j.bbamcr.2019.07.008
-
GPS 2.1: enhanced prediction of kinase-specific phosphorylation sites with an algorithm of motif length selectionProtein Engineering, Design & Selection 24:255–260.https://doi.org/10.1093/protein/gzq094
-
Pre-TCR signaling inactivates Notch1 transcription by antagonizing E2AGenes & Development 23:1665–1676.https://doi.org/10.1101/gad.1793709
-
Developmental gene networks: a triathlon on the course to T cell identityNature Reviews. Immunology 14:529–545.https://doi.org/10.1038/nri3702
Article and author information
Author details
Funding
Deutsche Forschungsgemeinschaft (NA 427/5-1)
- Anja C Nagel
Universität Hohenheim
- Anja C Nagel
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We are deeply grateful to Benedikt Häußling and Johannes Stökl (University of Bayreuth, Germany) for sending us all wasp species used in this study and for giving SD and LF a basic course in handling of the wasps. We thank Michèle Crozatier (Toulouse, France), David Hipfner (Montréal, Canada), Bruno Lemaitre (Lausanne, Switzerland), Sarah Bray (Cambridge, UK), Dieter Maier (Hohenheim, Germany), the Bloomington Drosophila Stock Center (BDSC, NIH P40OD018537) and the Vienna Drosophila Stock Center (VDRC) for numerous fly stocks. We acknowledge the Drosophila Genomics Resource Center (DGRC, NIH 2P40OD010949) for sending the Pkc53E cDNA, and the Developmental Studies Hybridoma Bank (DSHB), created by the NICHD of the NIH and maintained at the University of Iowa, Department of Biology, Iowa City, IA 52242 for providing several monoclonal antibodies. We thank Istvan Andó (Szeged, Hungary) for anti-Hemese antiserum, and Tina E Trenczek (Gießen, Germany) for anti-PPO1 antibodies. We very much acknowledge Franz Oswald (Ulm, Germany) and Tilman Borggrefe (Gießen, Germany) for the RBPjKO HeLa cell line. We are indebted to Lisa Lermer for her help in tissue preparations and screening of larvae and Janika Scharpf for the purification of the Pkc53E proteins. We are grateful to Armin Huber, Department of Biochemistry, for the use of the Apotome microscope, to Axel Schweickert, Department of Zoology, for use of the GloMax Discover Microplate Reader and to Jens Pfannstiel at the Mass Spectrometry Unit (Core Facility of the University of Hohenheim) for the MS/MS and ESI spectra. We thank Dieter Maier for helpful comments on the manuscript. Funding. This work was supported by a grant of the German Science Foundation DFG to ACN (NA 427/5-1) and by the University of Hohenheim. The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Version history
- Sent for peer review:
- Preprint posted:
- Reviewed Preprint version 1:
- Reviewed Preprint version 2:
- Version of Record published:
Cite all versions
You can cite all versions using the DOI https://doi.org/10.7554/eLife.89582. This DOI represents all versions, and will always resolve to the latest one.
Copyright
© 2023, Deichsel et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,150
- views
-
- 41
- downloads
-
- 2
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Cancer Biology
- Developmental Biology
Missense ‘hotspot’ mutations localized in six p53 codons account for 20% of TP53 mutations in human cancers. Hotspot p53 mutants have lost the tumor suppressive functions of the wildtype protein, but whether and how they may gain additional functions promoting tumorigenesis remain controversial. Here, we generated Trp53Y217C, a mouse model of the human hotspot mutant TP53Y220C. DNA damage responses were lost in Trp53Y217C/Y217C (Trp53YC/YC) cells, and Trp53YC/YC fibroblasts exhibited increased chromosome instability compared to Trp53-/- cells. Furthermore, Trp53YC/YC male mice died earlier than Trp53-/- males, with more aggressive thymic lymphomas. This correlated with an increased expression of inflammation-related genes in Trp53YC/YC thymic cells compared to Trp53-/- cells. Surprisingly, we recovered only one Trp53YC/YC female for 22 Trp53YC/YC males at weaning, a skewed distribution explained by a high frequency of Trp53YC/YC female embryos with exencephaly and the death of most Trp53YC/YC female neonates. Strikingly, however, when we treated pregnant females with the anti-inflammatory drug supformin (LCC-12), we observed a fivefold increase in the proportion of viable Trp53YC/YC weaned females in their progeny. Together, these data suggest that the p53Y217C mutation not only abrogates wildtype p53 functions but also promotes inflammation, with oncogenic effects in males and teratogenic effects in females.
-
- Developmental Biology
Stem cell self-renewal often relies on asymmetric fate determination governed by niche signals and/or cell-intrinsic factors but how these regulatory mechanisms cooperate to promote asymmetric fate decision remains poorly understood. In adult Drosophila midgut, asymmetric Notch (N) signaling inhibits intestinal stem cell (ISC) self-renewal by promoting ISC differentiation into enteroblast (EB). We have previously shown that epithelium-derived Bone Morphogenetic Protein (BMP) promotes ISC self-renewal by antagonizing N pathway activity (Tian and Jiang, 2014). Here, we show that loss of BMP signaling results in ectopic N pathway activity even when the N ligand Delta (Dl) is depleted, and that the N inhibitor Numb acts in parallel with BMP signaling to ensure a robust ISC self-renewal program. Although Numb is asymmetrically segregated in about 80% of dividing ISCs, its activity is largely dispensable for ISC fate determination under normal homeostasis. However, Numb becomes crucial for ISC self-renewal when BMP signaling is compromised. Whereas neither Mad RNA interference nor its hypomorphic mutation led to ISC loss, inactivation of Numb in these backgrounds resulted in stem cell loss due to precocious ISC-to-EB differentiation. Furthermore, we find that numb mutations resulted in stem cell loss during midgut regeneration in response to epithelial damage that causes fluctuation in BMP pathway activity, suggesting that the asymmetrical segregation of Numb into the future ISC may provide a fail-save mechanism for ISC self-renewal by offsetting BMP pathway fluctuation, which is important for ISC maintenance in regenerative guts.






