Immune Response: Investigating the role of vitamin D in asthma
Vitamin D deficiency has burgeoned into a major public health concern, exacerbated by dietary habits and pollution among other factors (Cui et al., 2023). While vitamin D is well known to be important for maintaining healthy bones, it has also been linked with various immune disorders, including asthma.
Supplements of vitamin D are being increasingly used to treat immune-related conditions. However, it remains unclear precisely how vitamin D is able to improve the outcome of these disorders (Scragg, 2018). Now, in eLife, Scott Weiss, Ardu Halu and co-workers – including Ayşe Kiliç as joint first author with Halu – report how vitamin D regulates an immune response that is a major contributor to asthma (Kiliç et al., 2023).
First, the team – who are based at Brigham and Women’s Hospital and Harvard Medical School, and institutes in Germany, Japan, Russia, and the United States – revisited the findings of a clinical trial called the Vitamin D Antenatal Asthma Reduction Trial (VDAART). In the trial, pregnant women who had a history of Asthma or allergies (or whose partner, the other biological parent, had a similar history), were given low or high doses of vitamin D during pregnancy. Analyses of the data found that higher vitamin D supplementation did not significantly reduce asthma in the offspring (Litonjua et al., 2016; Litonjua et al., 2020). However, a more nuanced reanalysis – which adjusted for baseline vitamin D levels to account for factors such as dietary intake – reported a reduced risk of asthma in the offspring of women who received a higher dose of vitamin D during pregnancy (Wolsk et al., 2017).
Kiliç et al. set out to find the genetic underpinnings of this protective effect, focusing their attention on chromosome 17, which contains regions strongly associated with asthma and other immune diseases (Bansal et al., 2021). Across chromosome 17 are sites where the receptor for vitamin D (known as VDR) can bind. Bioinformatic analysis revealed that some of these VDR binding sites overlapped with genetic variants associated with diseases triggered by the immune response mediated by T helper type 2 cells. These immune cells (known as Th2 for short) are a subset of white blood cells which respond proactively to invading pathogens like helminths, as well as recurrent exposures. Th2 cells act by producing inflammatory mediators as well as by modulating the activity of other cells in the immune system. They are also activated by allergens, such as mites and pollens, which can lead to allergic inflammation and disorders like asthma.
A detailed exploration of these overlapping regions suggested that VDR can trigger a cascade that regulates genes involved in the Th2 immune response. This genetic regulation could either promote or repress the Th2 response, depending on other genetic variants present in the proximity.
With a hypothesis established, the researchers tested their findings in mice which had been exposed to extracts from house dust mites to mimic the asthma phenotype (Figure 1A). They found that mice deficient in vitamin D or lacking VDRs displayed a more severe phenotype. Further experiments revealed that house dust mite exposure also caused the Th2 cells to express higher levels of VDR. When these Th2 cells were exposed to calcitriol (the active form of vitamin D), the VDRs bound to the calcitriol and migrated from the cytosol into the nucleus (Figure 1B).
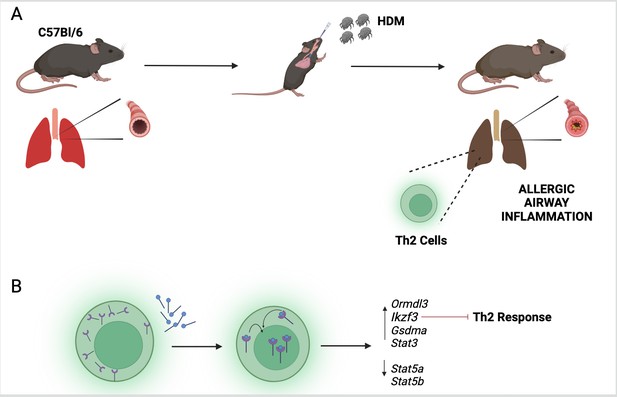
The vitamin D receptor and its role in inflammation of the airways.
(A) To mimic the symptoms of asthma, Kiliç et al. treated a lab-grown strain of mice (known as C57Bl/6) with extracts from house dust mites (HDM). This activated a group of immune cells called T helper type 2 (Th2; green) in the lungs of the mice, leading to inflammation of their airways. (B) Kiliç et al. found that exposure to dust mites also caused the Th2 cells to produce more vitamin D receptors (purple). When the cells were treated with calcitriol, the active form of vitamin D (blue circles with black lines), the receptors migrated from the cytosol to the nucleus. Once there, the receptor can regulate the expression of genes involved in the Th2 response, including the gene Ikzf3 which suppresses the inflammatory response triggered by Th2 cells.
© 2024, BioRender Inc. Figure 1 was created using BioRender.com, and is published under a CC BY-NC-ND license. Further reproductions must adhere to the terms of this license.
These findings suggest that VDR acts like a lock waiting for its key. Access to the key (vitamin D), and migration of the VDR into the nucleus, possibly unlocks the transcriptional regulation required to modulate the immune pathways. Furthermore, VDR expression was contingent upon baseline vitamin D levels, suggesting that this vitamin has both a preventive and therapeutic potential. Kiliç et al. also found that one of the genes that VDR regulates (called Ikzf3) is a major factor in suppressing the Th2 immune response (Figure 1B). This effect is likely mediated through the STAT signaling axis, which is a critical pathway regulating inflammation (Hu et al., 2021).
The study by Kiliç, Halu, Weiss and colleagues sheds light on how vitamin D offers protective benefits against asthma. However, several pathophysiological pathways can lead to the characteristic airway inflammation associated with asthma (Moore and Bleecker, 2014). Subgroup analysis during clinical trials, along with targeted exploration of relevant biomarkers, could help identify who would benefit most from vitamin D supplements. The latest findings also underscore the importance of vitamin D in a wider sense, as the Th2 response is associated with several other chronic inflammatory diseases.
That being said, it is essential to be mindful of the broader biological role of vitamin D, as it influences both the innate and the adaptive immune system via a number of different cell types (Colotta et al., 2017). This complexity may be why scientific literature on vitamin D is marred by contradictory findings: for instance, a previous study has even shown loss of VDR to be protective against asthma (Wittke et al., 2004). However, this complexity should not deter researchers from trying to develop a deeper mechanistic understanding of vitamin D effects. Such an understanding could, in the future, enable personalized treatment strategies for individuals with immune disorders such as asthma.
References
-
Advances in asthma geneticsAdvances in Genetics 107:1–32.https://doi.org/10.1016/bs.adgen.2020.11.001
-
Modulation of inflammatory and immune responses by vitamin DJournal of Autoimmunity 85:78–97.https://doi.org/10.1016/j.jaut.2017.07.007
-
The JAK/STAT signaling pathway: from bench to clinicSignal Transduction and Targeted Therapy 6:402.https://doi.org/10.1038/s41392-021-00791-1
-
Six-year follow-up of a trial of antenatal vitamin D for asthma reductionThe New England Journal of Medicine 382:525–533.https://doi.org/10.1056/NEJMoa1906137
-
Asthma heterogeneity and severity-why is comprehensive phenotyping important?Lancet Respiratory Medicine 2:10–11.https://doi.org/10.1016/S2213-2600(13)70288-1
-
Limitations of vitamin D supplementation trials: Why observational studies will continue to help determine the role of vitamin D in healthThe Journal of Steroid Biochemistry and Molecular Biology 177:6–9.https://doi.org/10.1016/j.jsbmb.2017.06.006
-
Vitamin D receptor-deficient mice fail to develop experimental allergic asthmaJournal of Immunology 173:3432–3436.https://doi.org/10.4049/jimmunol.173.5.3432
-
Vitamin D supplementation in pregnancy, prenatal 25(OH)D levels, race, and subsequent asthma or recurrent wheeze in offspring: Secondary analyses from the Vitamin D Antenatal Asthma Reduction TrialThe Journal of Allergy and Clinical Immunology 140:1423–1429.https://doi.org/10.1016/j.jaci.2017.01.013
Article and author information
Author details
Publication history
Copyright
© 2024, Sharma and Garg
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 639
- views
-
- 60
- downloads
-
- 1
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Genetics and Genomics
Genetic effects on complex traits may depend on context, such as age, sex, environmental exposures, or social settings. However, it remains often unclear if the extent of context dependency, or gene-by-environment interaction (GxE), merits more involved models than the additive model typically used to analyze data from genome-wide association studies (GWAS). Here, we suggest considering the utility of GxE models in GWAS as a trade-off between bias and variance parameters. In particular, we derive a decision rule for choosing between competing models for the estimation of allelic effects. The rule weighs the increased estimation noise when context is considered against the potential bias when context dependency is ignored. In the empirical example of GxSex in human physiology, the increased noise of context-specific estimation often outweighs the bias reduction, rendering GxE models less useful when variants are considered independently. However, for complex traits, we argue that the joint consideration of context dependency across many variants mitigates both noise and bias. As a result, polygenic GxE models can improve both estimation and trait prediction. Finally, we exemplify (using GxDiet effects on longevity in fruit flies) how analyses based on independently ascertained ‘top hits’ alone can be misleading, and that considering polygenic patterns of GxE can improve interpretation.
-
- Biochemistry and Chemical Biology
- Genetics and Genomics
Deep Mutational Scanning (DMS) is an emerging method to systematically test the functional consequences of thousands of sequence changes to a protein target in a single experiment. Because of its utility in interpreting both human variant effects and protein structure-function relationships, it holds substantial promise to improve drug discovery and clinical development. However, applications in this domain require improved experimental and analytical methods. To address this need, we report novel DMS methods to precisely and quantitatively interrogate disease-relevant mechanisms, protein-ligand interactions, and assess predicted response to drug treatment. Using these methods, we performed a DMS of the melanocortin-4 receptor (MC4R), a G-protein-coupled receptor (GPCR) implicated in obesity and an active target of drug development efforts. We assessed the effects of >6600 single amino acid substitutions on MC4R’s function across 18 distinct experimental conditions, resulting in >20 million unique measurements. From this, we identified variants that have unique effects on MC4R-mediated Gαs- and Gαq-signaling pathways, which could be used to design drugs that selectively bias MC4R’s activity. We also identified pathogenic variants that are likely amenable to a corrector therapy. Finally, we functionally characterized structural relationships that distinguish the binding of peptide versus small molecule ligands, which could guide compound optimization. Collectively, these results demonstrate that DMS is a powerful method to empower drug discovery and development.






